Topic 9 Acids and Bases
advertisement

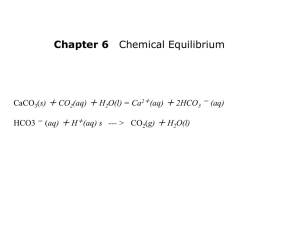
Topic 8 Acids and Bases 8.2 Properties of Acids and Bases (SL/HL) Acids = Substances that are able to produce H+ ions when dissolved in water. They cause litmus to go red, phenolphthalein to go colourless and methyl orange indicator to go red. Bases = Substances that can neutralise acids. Alkalis = Soluble bases that produce OH- ions when in water. They cause litmus to go blue, phenolphthalein to go pink and methyl orange to go yellow. Typical Reactions Give a fully balanced equation as an example to illustrate each type of neutralisation reaction. 1. Neutralisation with different types of base such as metal oxides or hydroxides. HNO3 + KOH HCl + NH4OH H2SO4 + CaO 2. Reaction with both soluble and insoluble carbonates and hydrogencarbonates. HCl + NaHCO3 3. Reaction with metals. H2SO4 + Mg -1- 8.1 Bronsted-Lowry Acids and Bases (SL/HL) Acids are substances which have a hydrogen ion concentration greater than 1 x 10 -7. In order to predict if a substance will be an acid or a base, we need a definition in terms of structure. Acids are proton H+ donars Bases are proton H+ acceptors For a species to act as an acid it must therefore contain a hydrogen atom attached by a bond which can be easily broken. H+ + Cl- HCl Will HF and HI be stronger or weaker acids than HCl? Draw ionic equations to illustrate the action of these acids. For a species to act as a base, it must contain a non-bonding e- pair which it can use to form a bond with a H+ ion. H+ + Donates H+ ion = acid OH- H2O Accepts H+ ion = base 2H+ + O2- H2O 2H+ + CO32- H2O + CO2 Conjugate Acids and Bases When an acid donates H+, the species produced is called a conjugate base as it now has the ability to accept a H+. When a base gains H+ the species produced is called a conjugate acid, as it now has the ability to donate a H+. H2SO4 Acid + ClBase HSO4conjugate base -2- + HCl conjugate acid HCl + Acid NH3 + Base H2O H3O+ Base conjugate acid H2O NH4+ Acid conjugate acid + Clconjugate base + OHconjugate base The stronger the acid, the weaker the conjugate base it produces. (HCl to Cl -) The weaker the acid, the stronger the conjugate base it producers. (H2O to OH-) CH3COOH + H3O+ H2O + CH3COO- What are the conjugate bases of the following acids?….H2SO4, HNO3, H2CO3, C2H5OH, C6H5OH, OH-, H2O. What are the conjugate acids of the following bases?…OH-, H2O, NH3, CO32-, HCO3HNO3, C2H5NH2. Label the Bronsted Lowry acid bases and conjugate acid bases in the following equation. HSO4- + H2NO3+ H2SO4 + HNO3 Amphiprotic (amphoteric) Substances (such as water) which are able to either donate or accept a H+ and so act as either an acid or a base is said to be amphiprotic. H3O+ OH- H2O Accepts H+ and so acts as a base -3- donates H+ and so acts as an acid. 8.1 Lewis Acids and Bases (SL/HL) Lewis extended the definition of acids and bases to include substances which do not contain hydrogen ions but which can still act as an acid or a base. An acid is an electron pair acceptor A base is an electron pair donor. E.g. 2 HCl + E.g. BF3 Mg + MgCl2 + H2 NH3 (BF3 NH3) Where H+/ BF3 have empty orbitals and so can act as a Lewis acids by accepting pairs of electrons. Mg/NH3 have non-bonded electron pairs that can donate and so can act as a Lewis bases. AlCl3 can also act as a Lewis acid as it has an empty bonding orbital. Bronsted lowry acids and bases can also be considered using the Lewis definition. E.g. H+ (can accept e- pair = acid) + OH- / O2- / CO32- (all have un-bonded epair that can be donated = base) Use ‘curly arrows to show the movement of electron pairs to illustrate the Lewis acid base nature of these reactions. HCl + NaOH H2SO4 + CuO HNO3 + CaCO3 -4- All transition metal compounds can act as Lewis acids since they all have empty orbitals. Any molecule that can form a dative covalent bond by donating a spare electron pair can act as a Lewis base. E.g. Cl-, H2O, CN-, OH-, NH3 etc. (called ligands) This means that all metal complexes which form (see Topic 3) are actually Lewis acid base reaction, where the metal is the acid and the ligand (H2O etc) is the base. E.g. Cu2+ + 6H2O Lewis acid [Cu(H2O)6]2+ Lewis base Use ‘curly’ arrows to show which of the following are Lewis acids and bases. Fe3+ + 6 CNCu2+ + 4 Cl- Ag+ 2 NH3 + TOK: Which of the two acid and base theories is the easiest to use? Why is there a need for two theories? -5- 8.3 Strong and Weak Acids and Bases. (SL/HL) Strong acids dissociate (ionise) completely in aqueous solution This means that all the molecules split up into ions in water. Examples of strong acids include hydrochloric, sulphuric and nitric. HCl H+ H2SO4 2H+ + SO42- HNO3 H+ NO3- + + Cl- Strong acids therefore have large concentrations of hydrogen ions in aqueous solution. Weak acids do not completely dissociate (ionise) in aqueous solution. This means that some of the molecules remain intact and do not split up into ions. The undissociated acid molecules and the dissociated ions form an equilibrium. Examples of weak acids include ethanoic and carbonic acids. CH3CO2H H+ + CH3CO2- H2CO3 H+ + HCO3- Weak acids therefore have lower concentrations of hydrogen ions in aqueous solution. -6- Strong and Weak Bases. Strong bases completely dissociate. Examples are group 1 metal hydroxides. Na+ + E.g. NaOH OH- Weak bases only partially dissociate. Examples are ammonia solution, carbonate solutions and amines (see Topic 10 organic chemistry). E.g. NH3 + CO32- H2O + 2H2O NH4+ + OH- CO2 + 2 OH- Experimental Determination of Strength of Acids. (SL/HL) Varying concentrations of H+ ions can be tested experimentally to see if the acid is strong or weak. 1. Conductivity: Since electrical conductivity relies on the movement of charges, strong acids have higher concentrations of H+ ions and so have more charges available. Strong acids are able to conduct electricity much better than weaker acids. This can be tested with a simple circuit containing an ammeter. 2. By Reaction with Carbonates: A simple indication of the strength of an acid can be given by comparing how violently it reacts with a carbonate. If equal amounts of two acids of the same concentration are added to calcium carbonate, the one that produces carbon dioxide at the greatest rate is the strongest. 3. pH: This is the measurement of H+ concentration. The lower pH value, the higher the concentration of H+, the stronger the acid. If two acids of equal concentration are tested with a pH meter the stronger one will give the lower value. Note: Weak acids are relatively safe, yet over a long period of time their effects become apparent. This happens in atmospheric acid rain. Weakly acidic rain slowly reacts with limestone and marble in buildings, and causes the leaching of important metal ions out of soil so that plants are unable to photosynthesize and grow. -7- 8.4 pH Scale (SL/HL) pH stands for power of hydrogen. The pH scale = 0 (very acidic, U.I red, stomach acid) 3-4 (weak acid, U.I orange, vinegar, lemon or orange juice) 7 (Neutral, U.I green, pure water) 9-10 (Weak alkali, U.I blue, washing powder) 14 (Strong alkali, U.I purple, oven cleaner) Where U.I is universal indicator used as a mix of indicators giving a wide range of colours. pH = Therefore if - log10 [H+] [H+] = 1 x 10-1 pH = 1 = 1 x 10-2 pH = 2 = 1 x 10 –5 pH = 5 = 1 x 10-7 (pure water) pH = 7 = 1 x 10-14 pH = 14 If the pH value changes by 1, this then actually means that the hydrogen ion concentration has changed by a factor of 10. A volume of 10cm3 of 0.1 mol dm-3 solution of HCl is diluted with water to 1000 cm3, what are the initial and final pH’s of the acid? What is the pH of a 5 cm3 sample of 0.01 mol dm-3 HNO3? Describe how the pH could be changed to pH = 6 by dilution. TOK: Why is it necessary to use a logarithmic scale to represent pH? What are the advantages? -8- 18.1 Calculations involving acids and bases (HL ONLY) pH Calculations pH = - log10[H+] The strength of a strong acid or base will be the same as its hydrogen ion concentration since both will completely ionise in solution So a 0.1 mol dm-3 solution of hydrochloric acid will have [H+] = 0.1 mol dm-3 Calculate the pH of the following a) b) c) d) 0.25 mol dm-3 HCl 2.5 HNO3 -2 1.5 x 10 HCl -5 1.4 x 10 HNO3 Calculate the hydrogen ion concentration of the following e) f) g) -9- pH pH pH = = = 2.5 6.4 4.5 18.1 The Ionic Product of Water (Kw) (HL ONLY) H+ H2O Equilibrium constant Kc + OH- [H]+ [OH]- / [H2O] = Since the equilibrium position is so far to the LEFT, the [H2O] concentration can be taken as being constant and so can be incorporated into the equilibrium constant. Ionic product of water Kw = [H]+ [OH](units = mol2 dm-6) Kw 1 x 10-14 mol2 dm-6 = at 298 K (25 degrees) room temperature Using Kw This can be used to determine the pH of alkaline solutions. E.g. Calculate the pH of a 0.1 mol dm-3 NaOH solution. [OH-] = 0.1 mol dm-3 since this is a strong alkali and fully ionises. Since Kw = 1 x 10-14 = [H+] [OH-] Using this to find the concentration of hydrogen ions [H+] = 1 x 10-14 / [ 0.1 ] [H+] = 1 x 10-13 pH 13 = E.g. 2 Calculate the pH of a 0.001 mol dm-3 solution of KOH E.g. 3 Calculate the pH of a 0.15 mol dm-3 solution of NaOH. E.g. 4 Calculate the pH of a 0.04 mol dm-3 solution of LiOH. Answer 2. pH 11 3. pH 13.2 - 10 - 4. pH 12.6 18.1 pH, pOH and pKw (HL ONLY) pOH is a measure of the hydroxide ion concentration on a log10 scale pOH = -log10 [OH-] pKw is a measure of the combined hydrogen and hydroxide concentrations on a log 10 scale. pKw = -log10 Kw Since Kw = 1 x 10-14 at room temperature pKw = 14 (at room temperature) This gives a useful and easily learnt relationship between pH and pOH pH + pOH = 14 E.g. Calculate the pOH of a solution of monoprotic acid of pH 4. pOH = 14 - 4 = 10 E.g. 2 Calculate the pOH of a monoprotic acid of concentration 0.02 mol dm-3 E.g. 3 Calculate the pOH and pH of a 0.03 mol dm-3 solution of LiOH. Answers 2)12.3 3) pH = 12.48 - 11 - pOH = 1.52 18.1 Kw and Temperature (HL ONLY) HEAT + H2O H+ + OH- This is an endothermic reaction. Increasing the temperature will therefore a) increase the amount of ionisation b) move the equilibrium to the RHS c) Increase both [H+] and [OH-]. Kw increases as the temperature increases Since [H+] increases, the pH will go down below 7, but the [OH-] will increases as well. This causes the pH to change, but the water to remain neutral. Water at a temperature above 25 degrees has a pH less than 7 Water at a temperature below 25 degrees has a pH greater than 7 E.g. Water at 70 degrees has a pH of 6.6 even though it remains neutral. Calculate the hydrogen ion concentration and pH of water if at a particular temperature Kw = 5 x 10-13 (Answer = pH 6.15) - 12 - 18.1 Equilibrium constants for weak acids and bases (HL ONLY) Weak acids and bases are only partially dissociated in water. Weak Acids H+(aq) HA(aq) + A-(aq) HA = acid molecule A- = salt ion Ka = [H+] [A-] / [HA] Ka = acid dissociation constant (the equilibrium constant for the dissociation of a weak acid.) If Ka is large = = = more dissociation equilibrium further to RHS stronger acid If Ka is small = = = less dissociation equilibrium further to LHS weaker acid Since the values for Ka can have such a large range, the numbers are usually given as log10. pKa = - log10 [Ka] A pKa of 1 or 2 would therefore be a very strong acid. A pKa of 4 or 5 would be a weaker acid such as ethanoic acid. A pKa of 13 or 14 would be a very weak acid such as ethanol. - 13 - Weak Bases (HL ONLY) B + BH+ H2O + B = Base Kb [BH+] [OH-] / [B] = And pKb = - log10 Kb So that strong bases have a pKb of 1 or 2. Weaker bases (such as ammonia) have a pKb of 4 or 5. Very weak bases have pKb values of 9 or 10. Use the data booklet to: a) Calculate the Ka for ethanoic acid. b) Calculate the Kb for ammonia. - 14 - OH- 18.1 Calculations Involving Weak Acids and Bases (HL ONLY) H+(aq) HA(aq) Ka + A-(aq) [H+] [A-] / [HA] = If the initial concentration of the weak acid HA = a The equilibrium concentration of H+ and OH- = x Then the equilibrium concentration of HA = (a-x) This gives the equilibrium expression Ka = x2 / (a – x) Since x is so much smaller than a for a weak acid, we can assume that (a – x) = a This gives: Ka = x2 / a Where x is the equilibrium concentration of H+ calculated from the pH of the solution and a is the initial concentration of the weak acid. 1. Calculating pH Calculate the pH of a 0.1 mol dm-3 solution of a weak acid of pKa 4.2. pKa = 4.2 pKa = - log10 Ka Ka = 6.31 x 10-5 mol dm-3 Ka = x2 / a 6.31 x 10-5 = x2 / 0.1 x = 2.51 x 10-3 mol dm-3 pH = - log10 [2.51 x 10-3] pH = 2.6 - 15 - 2. Calculating concentrations Calculate the concentration of a weak acid HF of pH 2 where Ka = 6.76 x 10-4 mol dm-3 pH = 2 [H+] = therefore 2 = - log10 [H+] [F-] = Ka = [H+] [F-] / [HF] Ka = x2 / a 6.76 x 10-4 = therefore [HF] = 1 x 10-2 mol dm-3 = x (1 x 10-2)2 / [HF] 0.148 mol dm-3 3. Calculating Ka Calculate the dissociation constant Ka for a weak acid HA if a 0.01 mol dm-3 solution has a pH of 3.1. 0.01 mol dm-3 [HA] = pH = 3.1 therefore [H+] = 7.94 x 10-4 mol dm-3 Ka = x2 / a = (7.94 x 10-4)2 / (0.01) = 6.31 x 10-5 mol dm-3 = a 3.1 = - log10 [H+] = x What is the value for pKa? Use the data booklet to identify this organic acid. - 16 - Weak Bases (HL ONLY) B + BH+ H2O + OH- Kb = [BH+] [OH-] / [ B ] Note the [H2O] concentration is very large and therefore taken as constant. It is incorporated into the K b value. Kb = y2 / b Same as acids! Note: Kw = Ka x Kb = 1 x 10-14 so that: pKa + pKb = 14 Sneaky eh? Questions 1. Calculate the pH of a 0.1 mol dm-3 solution of ethanoic acid if Ka is 1.8 x 10-5 mol dm-3. 2. Find Ka of a weak acid of concentration 0.02 mol dm-3 if its pH is 3.9. 3. Find the concentration of a weak acid of pH 4.5 if Ka = 4.1 x 10-6 mol dm-3 4. Calculate the pH of a weak base of concentration 0.01 mol dm-3if Kb = 1.8 x 10-5 mol dm-3. 5. If the pH of a weak base is 10, and its concentration is 3 x 10 -2, calculate the pKb of this base. 6. If the pKa of methylamine (a weak base) is 10.64, and it’s pH is 10.8, what will be it’s concentration? Answers = 1) pH 2.87 2) 7.92 x 10-7mol dm-3 3) 2.44 x 10-4 mol dm-3 4) pH 10.65. 5) 6.48 6) 9.13 x 10-4 mol dm-3 - 17 - 18.2 Buffer Solutions (HL ONLY) Buffers resist changes in pH when small amounts of acids or bases are added. Acidic Buffers: pH remains constant and acidic with a pH of less than 7. Prepared by mixing a weak acid with an aqueous solution of a salt of that weak acid. E.g. Weak acid = ethanoic acid (CH3CO2H) Mixed with salt of weak acid = sodium ethanoate (CH3CO2Na). The weak acid partially dissociates and sets up an equilibrium that obeys Le Chateliers principle: CH3COOH CH3COO- + H+ Addition of a small amount of acid H+ shifts the equilibrium position to the LHS so that the H+ concentration stays constant and the pH remains the same. Addition of a small amount of base (OH-) reacts with the H+ ions and causes the equilibrium position to shift to the RHS. The H+ concentration and also the pH therefore remain constant. Ideally there should be equal concentrations of weak acid (CH3COOH) and the salt of the weak acid (CH3COO-) so that the solution can buffer equally in both directions. Note: A buffer can also be made by neutralising excess ethanoic acid with sodium hydroxide as the limiting reagent: CH3CO2H + (excess) 0.2 moles NaOH CH3CO2Na + CH3CO2H + H2O (limits) 0.1 moles (salt) 0.1 moles - 18 - (weak acid) 0.1 moles Basic Buffers pH remains constant and basic with a pH greater than 7. Prepared by mixing a weak base with the salt of a weak base. E.g. Weak base = Ammonia NH4OH Salt of a weak base = Ammonium chloride NH4Cl The weak base partially dissociates and sets up an equilibrium that obeys Le Chateliers principle: NH4+ NH4OH + OH- Small additions of acid will react with OH- and shift the equilibrium position to the RHS so that the pH does not change. Small additions of OH- will shift the equilibrium to the LHS. pH stays constant. Ideally there should be equal concentrations of weak base (NH4OH) and the salt of the weak acid (NH4+ ) so that the solution can buffer equally in both directions. Note: The salt can be either added directly or made by neutralising excess ammonia with some hydrochloric acid: NH3 + HCl 0.2 mole NH4Cl 0.1mole 0.1 mole + NH3 0.1 mole Examples of Natural Buffers: Blood Blood contains a very important buffer which makes it function properly. In the lungs, [O2] is high, eq shifts to RHS causing O2 to combine with haemoglobin molecules. HHb + O2 H+ + HbO2- In the muscles [O2] is low, eq shifts to LHS causing O2 to be released. Changes in concentration of H+ will affect whether the blood is able to function correctly. The blood therefore has a buffer in it to make sure that that the pH cannot alter significantly. - 19 - 18.2 Buffer Calculations (HL ONLY) Since buffers are fundamentally weak acids and bases, the calculations are very similar E.g. H+ CH3COOH + CH3COO- The essential difference is that a large amount of the salt is also added to the solution and completely ionises, which makes the concentration of the salt ion (CH3COO-) different to the concentration of hydrogen ions. The assumption made is that since the equilibrium of the weak acid/base is so far to the LHS, that the ionisation of the acid is so small that concentration of the salt ion comes only from the salt and not from the dissociation of the weak acid. E.g. [CH3COO-] = from the concentration of salt solution [H+] = from the pH of the weak acid [CH3COOH] = From the initial concentration of the weak acid. E.g. A buffer solution was prepared by adding 2.05g of sodium ethanoate to 500cm3 of ethanoic acid of concentration 0.2 mol dm-3. Calculate the pH of the resulting buffer. pKa ethanoic acid = 4.76. pKa = - log10 Ka = 1.74 x 10-5 Moles of CH3COONa = m / Mr = 2.05 / 82 = 0.025 [CH3COO-] = n / V = 0.025 / 0.5 dm3 = 0.05 mol dm-3 [CH3COOH] = 0.2 mol dm-3 Using eq equation: 1.74 x 10-5 = Ka = [H+] [CH3COO-] / [ CH3COOH] [H+] [0.05] / [ 0.2] [H+] = 6.96 x 10 –5 mol dm-3 pH 4.16 = Extension: How could this buffer solution be made more acidic? - 20 - E.g. 2 If a buffer is made by mixing 0.2 moles of sodium ethanoate into 0.5 dm3 of ethanoic acid of concentration 0.1 mol dm-3, then what will be the resulting pH of the buffer?(pKa = 4.76) E.g.3 The weak acid propanoic acid (pKa = 4.87) can be used to make a buffer if it is mixed with it’s salt sodium propanoate (C2H5COONa). Calculate the mass of salt that must be dissolved in 0.25 dm3 of 1 mol dm-3 propanoic acid to give a buffer of pH 4.87. Answers: 2. pH = 5.36 3. 24 g Note: Most buffers are prepared so that they can shift equally to the LHS and to the RHS. This means that usually the concentration of the weak acid equals the concentration of the salt. [weak acid] = [salt] (E.g. [CH3COOH] = [CH3COO-] ) So that they cancel each other out. This means that often Ka = [H+] And so (often) in an ideal buffer: pKa = pH E.g. If 40 cm3 of 0.1 mol dm-3 ethanoic acid is mixed with 20 cm3 of 0.1 mol dm-3 sodium hydroxide, what would the pH of the buffer be if Ka = 1.74 x 10-5 mol dm-3? E.g. If 100cm3 of 0.5 mol dm HCl were mixed with 200cm3of ammonia solution. Calculate the pH of the resulting buffer. (pKa for ammonia = 4.75) Answer 1. pH = 4.76 2. pH = 9.25 - 21 - 18.3 Salt Hydrolysis Salts are derived from acids that have been neutralised by a base. Salts made from strong acids and bases will be neutral. If the acid and base involved are both strong, the resulting salt will be neutral. E.g. Cl-, SO42-, NO3- are conjugate bases of strong acids and so will be only very weak bases. E.g. Na+, K+ are conjugate acids of strong bases and so will be only very weak acids. NaCl, KNO3 etc will therefore be neutral. Salts made from weak acids and strong bases will be alkaline. E.g. CH3COOH is a weak acid and therefore gives a conjugate base CH3COO- which is a strong. CH3COONa will therefore be alkaline. Salts made from strong acids and weak bases will be acidic. E.g. NH3 is a weak base and therefore gives a conjugate acid NH4+ which is a strong. NH4Cl will therefore be acidic. Salts made from weak acids and bases will be neutral. This is because the conjugate acid and base produced will both be strong. However they will cancel each other out. CH3COONH4 will therefore be neutral. 1. 2. 3. 4. 5. 6. 7. 8. KCl Na2SO4 CH3CO2K NH4NO3 Na2CO3 (NH4)2CO3 NaNO3 (NH4)2SO4 - 22 - Metal Salt Solutions: Some metal salt solutions may be acidic due to the charge on the metal ion. +1 metal ions in solution tend to be neutral, but due to the increased charge on the metal ion, +2 and then +3 metal ion solutions increase in acidity. E.g. AlCl3 dissolved in water gives a complex ion Al(H2O)63+ The Al3+ is acting as Lewis acid due to its empty orbitals. H2O is acting as a Lewis base due to it donating electron pairs to form 6 co-ordinate bonds. Since the Al3+ is so highly charged it can cause the water molecules to break apart (called hydrolysis) Al(H2O)63+ Al(H2O)3(OH)3 + 3H+ Since the Al causes the water to split and H+ ions are made, then this makes it an acidic solution. This is an equilibrium reaction. Al(OH)3 is therefore also amphiprotic as it can either gain H+ ions to reform Al(H2O)6 and so act as a base. Al(H2O)3(OH)3 + 3H+ Al(H2O)6 or it can continue to lose H+ ions and so act as an acid. Al(H2O)3(OH)3 Al(H2O)2(OH)4- + H+ Show an equation to explain why copper (II) salts tend to be slightly acidic solutions. Show an equation to explain why iron III salts tend to be more acidic solutions. Predict the acidity of the following metal salts based on their charge density. 1. FeCl3 4. MgSO4 7. Cu(NO3)2 2. FeSO4 5. CrCl3 8. Al2(SO4)3 - 23 - 3. KNO3 6. NaCl 18.4 Acid and Base Titrations (HL ONLY) 1. Strong Acid (HCl) and Strong Base (NaOH). [HCl] = [NaOH] = 0.1 mol dm-3 Point of inflexion/equivalence point. This is the steepest point of the graph where the solution becomes neutral. pH Volume of 0.1 mol dm-3 NaOH (cm3) Since HCl is a strong acid; Initially [H+] = 1 x 10-1 mol dm-3; pH of the acid = 1. When 90% of the HCl has been neutralised, only 10% remains, the [H+] = 0.01 mol dm-3 (decrease by a factor of 10) pH = 2. When 99% of the HCl has been neutralised, only 1% remains. The [H+] = 0.001 mol dm-3 (decrease by another factor of 10) pH = 3. This shows why the pH remains low up until the final drop of HCl has been neutralised. When HCl is completely neutralised (with 10cm3 0.1 mol dm-3 NaOH), only salt and water are present in solution, causing a rapid increase in pH = 7; [H+] = 1 x 10-7 mol dm-3. The hydrogen ions present are due to the ionic product of water (Kw). The equivalence point is at pH 7 and has a large range. Many indicators can be used in this pH range. When a small excess of NaOH is added, the [OH-] rises quickly causing [H+] to rapidly decrease. The pH increases rapidly. [OH-] = 1 x 10-1 [H+] = 1 x 10-13 mol dm-3 pH = 13. - 24 - 2. Weak acid (CH3COOH) and strong base (NaOH). [CH3COOH] = [NaOH] = 0.1 moldm-3 pH Volume of 0.1 mol dm-3 NaOH (cm3) 10 cm3 of a weak acid will still require 10 cm3 of strong base of the same concentration for neutralisation. Since the acid is only partially ionised the initial concentration of H+ is lower and so the starting pH is higher (3 or 4 ). The rise in pH is gradual up to the end point. This is because the salt produced is the salt of a weak acid, which will therefore act as a buffer with the remaining weak acid. E.g. CH3COOH + NaOH CH3COONa + CH3COOH(excess) + H2O It will therefore resist the change in pH when the strong base is added until all the acid has been used up. The equivalence/point of inflexion occurs higher (in the region pH 9-10). An ideal indicator for this type of reaction should change colour in this region (phenolphthalein). Label the buffered region and the equivalence point on your graph. When all the acid has been neutralised, the pH rapidly increases to that of a strong base (pH 13). - 25 - 3. Strong Acid (HCl) and Weak Base (NH4OH) [HCl] = [NH4OH] = 0.1 moldm-3 pH Volume of 0.1 mol dm-3 NH4OH (cm3) pH will start low (pH = 1) The equivalence point is lower (in the range pH 3 to 7). An ideal indicator for this type of reaction should change colour in this region (methyl orange). Once all the acid particles have been used up, there will be a weak base and the salt of a weak base present. This will therefore act as a buffer and will resist any further change in pH. The pH will therefore only rise slowly to 10 or 11. Label the buffered region and the equivalence point on your graph. - 26 - 4. Weak Acid (CH3COOH) and Weak Base (NH4OH). [CH3COOH] = [NH4OH] = 0.1 moldm-3 pH Volume of 0.1 mol dm-3 NH4OH (cm3) 10 cm3 of a weak acid will still require 10 cm3 of weak base of the same concentration for neutralisation. Since the acid is only partially ionised the initial concentration of H+ is lower and so the starting pH is higher (3 or 4 ). The neutralisation produces the salt of a weak acid and so this region is buffered. The equivalence point occurs at pH 7 but is of a very narrow range. Most indicators cannot be used effectively. When the weak base is in excess it also buffers with the salt. The pH therefore only rises slowly to pH 10 or 11. Indicate on your graph the equivalence point and buffered regions. - 27 - 18.6 Indicators (HL ONLY) All indicators are weak acids and bases. The indicator must be one colour as a weak acid/base and then a different colour when it has formed its conjugate acid/base. e.g. Weak acid indicator such as methyl orange (pKa 3.7) H+ HIn (Red) + In(Yellow) Addition of a small amount of H+ will shift eq. to LHS causing the substance to go red. Addition of a small amount of base will shift eq. to RHS causing the substance to go yellow. Due to its pKa value, methyl orange will change colour at a pH of 3.7. (This change in colour happens when the concentration of [HIn] is equal to [In-]. At this point Ka = [H+] and so pKa = pH. See later notes) This makes methyl orange an ideal indicator for titrations involving strong acids and weak bases since the equivalence point is lower (between 3 and 7). e.g. Weak base indicator such as phenolpthalein (pKa 9.3) In + H2O (pink) InH+ + OH- (colourless) Addition of a small amount of H+ will shift eq. to RHS and solution will be colourless. Addition of small amount of base will shift eq. to LHS and solution will be pink. Due to pKa value, phenolpthalein will change colour at a pH of 9.3. This makes phenolpthalein ideal for use in titrations involving weak acids and strong bases since the equivalence point is higher (between 8 and 10) - 28 - 18.5 Calculations Involving Indicators E.g For a weak acid indicator (pKa = 3.7) H+ HIn + (Red) In(Yellow) Kin = [H+] [In-] / [HIn] The point at which an indicator changes colour will be when the concentrations of both colours are equal. i.e. [red] = [yellow] When the concentration [red] exceeds [yellow] then the indicator changes from red to yellow, or vice-versa. At this point: [HIn] = [In-] Cancelling out from the above equation giving: Kin = [H+] pKin = pH This explains how it is possible to predict at which pH range an indicator will change colour. (Note also similarities with buffer calculations where pH (of buffer) = pKa (of the weak acid).) Questions: 1. At what pH range does methyl orange change colour? (pKa = 3.7) 2. At what pH range does phenolphthalein change colour (pKb = 9.3) 3. Label the pH, pKa or pKb on the appropriate titration graphs for these two indicators. 4. Give an example from the data booklet of another indicator that could be used for a strong acid/weak base titration. Explain. 5. Give an example from the data booklet of another indicator that could be used for a weak acid/strong base titration. Explain. 6. Which indicator would be most effective to determine the end point of a weak acid/weak base titration? Explain. - 29 -