Strong Acid + Base – pH, pOH calculations pH =
advertisement

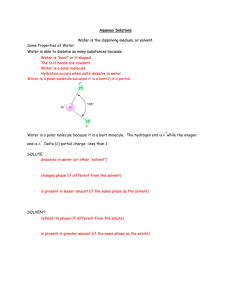
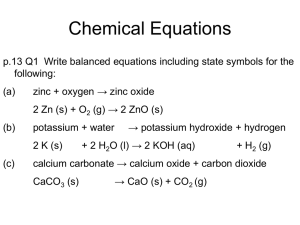
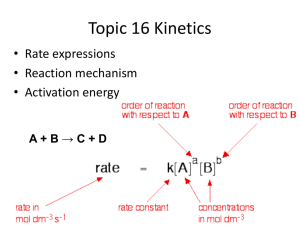
Strong Acid + Base – pH, pOH calculations pH = -log10 [H+] What do the square brackets [H+] represent? H+ is a hydrogen ion, but these do not really exist in an aqueous environment. What is the alternative ion that is present? Do you know how to use your calculator? a) Find the pH if [H+] = 0.1 mol dm-3 b) find the pH if [H+] = 5.6 x 10-4 mol dm-3 You should get the answer = 1 You should get the answer = 3.25 How does your calculator do the opposite of log? Sometimes it is shift+log sometimes separate button 10x To find the [H+] having been given the pH, [H+] = 10-pH c) Find the [H+] if pH = 2 d) find the [H+] if pH = 5.65 You should get the answer = 0.01 You should get the anwer = 2.2 x10-6 If you did – GREAT – move on….. otherwise – you need to speak to Mrs Higgins in order to sort out how to do this on your calculator! Complete these problems: 1) Convert the following hydrogen ion concentrations (mol dm-3) into pHs; a) 0.0100 b) 0.0250 c) 3.00 x10-4 d) 1.00 x 10-7 e) 7.50 x10-10 2) Convert the following pHs into hydrogen ion concentrations in mol dm-3 a) 3.42 b) 1.20 c) 5.65 d) 8.40 e) 13.0 Finding the pH of a strong acid Define an acid: A strong acid is one which breaks up fully into its ions when it is added to water Write an equation to show this for each of the following strong acids HCl H2SO4 HNO3 Use your equations above to work out the following: a) b) c) d) e) f) g) h) How many H+ ions would be produced from 10 HCl molecules splitting up? How many H+ ions would be produced from 10 HNO3 molecules splitting up? How many H+ ions would be produced from 10 H2SO4 molecules splitting up? How many H+ ions would there be in 1 litre of water if there were 15 HCl molecules added to a litre of water? How many mols of H+ ions would there be per litre if 15 mols of HNO3 were added? How many mols of H+ ions would there be per litre if 15 mols of H2SO4 were added? What would be the concentration of H+ ions, [H+], in HCl with a concentration of 0.01 mol dm-3? What would be [H+] in 1.50 mol dm-3 H2SO4 For strong acids the [H+] is directly related to the concentration of the original acid. It has all split up to make hydrogen ions. 1) Calculate the pHs of the following strong acids? a) 0.0300 mol dm-3 HCl b) 0.00500 mol dm-3 H2SO4 c) 0.120 mol dm-3 HNO3 2) Calculate the concentration of the following strong acids from their pHs: a) HCl with a pH of 0.70 b) Sulphuric acid with a pH of 1.5 c) Nitric acid with a pH of 2.0