Stoichiometry LP
advertisement

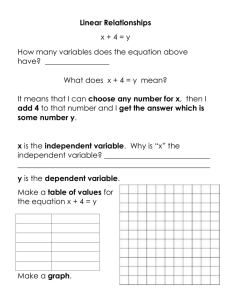
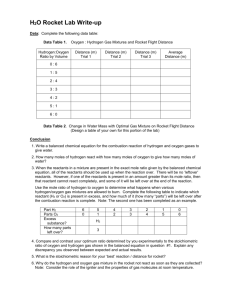
LESSON PLAN TEMPLATE – Classroom Interactions Big Idea/ Enduring Understanding for the lesson: Stoichiometry: Law of Conservation of mass, qualitative experiment TEKS for lesson: (6) Science concepts. The student knows and understands the historical development of atomic theory. The student is expected to: (E) express the arrangement of electrons in atoms through electron configurations and Lewis valence electron dot structures. (7) Science concepts. The student knows how atoms form ionic, metallic, and covalent bonds. The student is expected to: (C) construct electron dot formulas to illustrate ionic and covalent bonds; (8) Science concepts. The student can quantify the changes that occur during chemical reactions. The student is expected to: (D) use the law of conservation of mass to write and balance chemical equations; and (E) perform stoichiometric calculations, including determination of mass relationships between reactants and products (10) Science concepts. The student understands and can apply the factors that influence the behavior of solutions. The student is expected to: (A) describe the unique role of water in chemical and biological systems; Objective/s- Write objective/s in SWBAT form… The SWBAT: Assessment: Completed worksheets, participation in discussion, and analysis of predictions. Formative during lab time. Overview of Activities: 15-20min: Introduction/Review/Vocabulary – Explain or review concepts of stoichiometry. [Coefficients, subscripts, reactants/products, reverse reactions, association/dissociation, Law of Conservation of Mass.] 25-35min: Experiment – Splitting of water using electrochemistry. [No knowledge of electrochemistry needed.] Qualitative explanation of stoichiometry, standard states, and use of experimental design and lab ethic. 10-15min (or assigned homework): Analysis/Discussion of results – Visible products explain stoichiometry. LESSON EPISODES (In segments. No set number.) Estimated Time ~1520min Segment title/ description Introductio n and real world application What teacher is doing step by step (Include specific questions that the teacher will ask as well as sample problems) What students are doing (consider transitions to the next segment and grouping) Present students with the concepts behind baking. Set up an “equation” with the ingredients, the reactants, of chocolate chip cookies added together to get an x amount of cookie products. ex: _eggs+_flour+_sugar+_chocolate chips+_butter->_cookies Students are taking notes in their designated spirals or folders. Start with stating that basic amount of ingredients that make 12 cookies. Fill in the blanks appropriately, they do not have to exact it’s all hypothetical, and explain the use of the coefficients. [Make sure to note that the reaction can happen in reverse, cookies can be ‘dissociated’ to regain the reactants. Not always true for all equations but for the sake of this example it could be helpful.] Teacher: So I want to make x amount of cookies. What would I do if I wanted to get x amount of eggs? X amount of sugar? Student: Go backwards? Divide? That’s impossible. Teacher: Maybe when making cookies, but a lot of chemical equations can actually go in reverse. Coefficients are the numbers next to a reactant or product that tells how many are needed or created for a reaction. Once this is understood, the class should have a brief grasp on reactants, products, and coefficients. Then explain what would happen to the reactants if we only made 6 cookies? 24 cookies? 18 cookies? Let the students think pair share at their tables or with a partner for about 3 minutes and ask one student from Teacher: What happens to the number of reactants as the number of products are reduced? Student: There are less than before. They also decrease. It would stay the same, you would just have extra dough. each different group (3 total) to come to the board and show their answer. This will spark discussion of how the amount of product can be controlled by the amount of reactants. To introduce subscripts, replace sugar with actual chemical formula of glucose in the recipe. _eggs+_flour+_C6H12O6+_chocolate chips+_butter->_cookies Explain how the coefficient is then multiplied throughout each element if that element has any. For any without subscripts, it is assumed there is only one of those elements. End introduction by presenting real chemistry equations and starting discussion of how we can see how much product is getting made. Since the product isn’t as visible as the number of cookies that change, there has to exist some knowledge about the components of the reactants and equation. Bring up Law of Conservation of Mass. (Should be review.) Some example equations: 𝑁𝐻4 ↔ 𝑁𝐻3 + 𝐻 + 6𝐶𝑂2 + 6𝐻2 𝑂 → 𝐶6 𝐻12 𝑂6 + 6𝑂2 Allow students to then work on Supplement A. (~5min) Resources for this segment & Teacher: What is the subscript? Student: The number that comes after the element The subscript identifies the molecule. Teacher: What is the coefficient? Student: The number before the molecule The coefficient identifies how many molecules are present. Teacher: What is the difference between a coefficient and a subscript? Student: You can change the coefficient, but you cannot change the subscript Terms to know: Word bank could be used here. dissociation association/synthesis/recombination coefficients reactants products reverse reaction subscripts Lass of Conservation of Mass Supplement A worksheets, chalkboard/whiteboard/doc cam. Optional: Word bank, actual cookies for engagement. SAFETY considerati ons ~3035min Water splitting experiment The reaction of interest today is the splitting, or dissociation of water into its original elements. _𝐻2 𝑂(𝑙) ↔ _𝑂2 (𝑔) + _𝐻2 (𝑔) Make note of the physical states of water and hydrogen and oxygen. Also make note of the double headed arrow to show that it is a reversible reaction. Sort students into their lab groups. Explain that the stoichiometry of this equation is incomplete and the lab today will show qualitatively how this reaction proceeds. Before students can be released to start the lab, they must preset their finished balanced reaction to show their understanding of how coefficients work. They must also predict what they think will show in the experiment. [This can be variable, just allows students to make their own ideas and assumptions. Right or wrong still helps understanding after experiment.] Teacher: Does anyone know the reaction for the association or dissociation of water? What is the reaction? [Either form is applicable.] Student: It’s made up of hydrogen and oxygen. You can’t split water, it’s pure. Teacher: If we can agree that hydrogen and oxygen make up water, how would I write that equation? Student: 𝐻2 𝑂 → 𝐻 + 𝑂 (Just one possible misconception.) Teacher: Can hydrogen and oxygen simply exist as lone elements though? What do we know about standard states of elements? Student: Oxygen and hydrogen exist as diatomic gases in their standard states. Teacher: Show assigned lab groups/tables. Preferable with 3 or less in each group. In your lab notebooks, decide in your groups the correct final equation and make a prediction, at least 2 sentences, about what you think you will see. [Pass out supplement B at lab tables.] Student: If we’re making gases, how can we see the products? Teacher: I’m glad you asked, here we have a short video to show the lab. [Show youtube video, make sure to not show results.] Present YouTube video Using the supplement B as a guide and the video as background, the lab groups should be able to follow the instructions. Trouble may Teacher: Make sure to take observations and notes of anything you may think are important! occur in filling up the vials with water, so help may be needed. Other steps should not need much assistance. Resources for this segment & SAFETY considerati ons ~1015min or homework Supplement B worksheets, lab notebooks for observations/predictions, YouTube video, lab kits, outlets (12V adapters), water, 500mL beakers, sodium sulfate (20g per kit), and clip leads. Goggles mandatory. Gloves optional. Go over lab safety rules: Goggles AT ALL TIMES, no exceptions! Hydrogen is safe in quantities generated in this experiment (<15mL) and will dissipate without effect, but H 2 should not be generated in any great quantity than that suggested here! Analysis/ Discussion Have students close down lab, should take less than five minutes. Return to desks and have them answer analysis questions. Can be used as an exit ticket or as assigned homework. If assigning analysis as homework, start class discussion. If students are confused or struggled too much with lab, it is preferred to end with discussion so they have basis to answer the homework. Any misconceptions or questions should be answered. Some example discussion topics are listed. Could be more in depth with stoichiometry and law of conservation of math. Take which direction the class is lacking in. DISCUSSION: Teacher: I need to someone to come write their equation on the board that they used in their experiment. Student: If they write the correct equation, ask if anyone got a different answer. If they did, ask other class members what is different. If they write the incorrect equation, ask another student to write their equation too. There shouldn’t be much variation, but having multiple students write on the board will put less emphasis on the student that may have been wrong. Teacher: So the correct final equation is – 2𝐻2 𝑂(𝑙) ↔ 𝑂2 (𝑔) + 2𝐻2 (𝑔) How many of each element are there on each side? Remember that products must equal reactants. For advanced classes: Have students write a full lab report. With abstract, experimental design, results and conclusion. Resources for this segment & SAFETY considerati ons Student: 4 hydrogen and 2 oxygen. Teacher: In comparison to oxygen molecules, how many more hydrogen molecules are there? Student: There are two more hydrogens. Hydrogens are double the amount of oxygen. Teacher: Did we see this in the experiment? Can someone explain what they saw? Student: There was more gas in one tube than in the other. It looked about twice as much. I couldn’t really tell. Teacher: So we agreed that our equation implies that there are two times more hydrogen than oxygen. What tube do you think was producing hydrogen? Which was producing oxygen? Analysis worksheets. Make sure that all ac adapters are unplugged and the water is disposed of properly. Supplement A (ANSWERS) Warm-Up 1. In one sentence, what does the Law of Conservation of Mass state? Product = Reactants Mass cannot be created nor destroyed The law implies that mass can neither be created nor destroyed. 2. Label the chemical equation below using the following words: Reactants Products Coefficients Subscript How many of each element are in each side of the photosynthesis equation? 6𝐶𝑂2 + 6𝐻2 𝑂 → 𝐶6 𝐻12 𝑂6 + 6𝑂2 C H O #6 #12 #18 3. SUPPLEMENT B H2 from H2O Laboratory Procedure Materials and Supplies: Amperostat electrostation 12V AC adapters Tap water and sodium sulfate (Na2SO4) Electrode assembly, and tall 500 mL Beaker Clip leads (5) Procedure: 1. Fill the tall beaker about 3/4 full with deionized or tap water, and dissolve about 20 g of Na2SO4. 2. Make sure the ON/OFF switch is set to OFF, and the dial is set to MIN. Using the included clip leads: A) connect battery #1 (+) terminal to the Battery (+) terminal on the electrostation B) connect Battery #2 (-) terminal to the Battery (-) on the electrostation C) between the two batteries, connect Battery #1 (-) to Battery #2 (+) to complete the circuit. 3. Fill the electrode assembly with the solution, and then submerge the electrode assembly by inverting them quickly at 45° angle into the beaker which is held also at 45°. 4. Again using clip leads, connect the two electrodes to the "(+) Electrode (-)" leads on the electrostation (in any order, it doesn't matter). 5. Note the water level in each electrode test tube, and write it down in your pre-made data table. 6. Turn the electrostation ON, and adjust the dial so that the meter reads 50, 100, 150 or 200 mA (depending on your instructor's direction). 7. Watch as gaseous bubbles evolve from the very tips of the electrodes. Run the reaction for 5-15 min as directed by your instructor. 8. In your data table, write down the final water level in each electrode. 9. Empty the solution from the test-tube electrodes back into the beaker, and re-do the experiment under different conditions (different time, different mA setting). Data and Observations: Effect of Reaction Time on H2 Production Set to 200 mA Time of Reaction (min) 5 Tube #1: initial Reading (mLi) Tube #1: final Reading (mLf) Gas produced (mLf i) Tube #2: Initial Reading (mLi) 10 15 20 Tube #2: Initial Reading (mLf) Gas produced (mLf i) Data Analysis: [1] In your own words, describe what happened with water during the experiment. [2] Based on the Law of Conservation of Mass, which tube was generating H2? Which tube was generating O2? Why? [3] Since you know the basics of a chemical equation, write down the chemical equation that took place in this experiment. If this equation was different from your original prediction, what was different? Why is this version correct? If your prediction was right, explain why.