Balancing Chemical Equations
advertisement
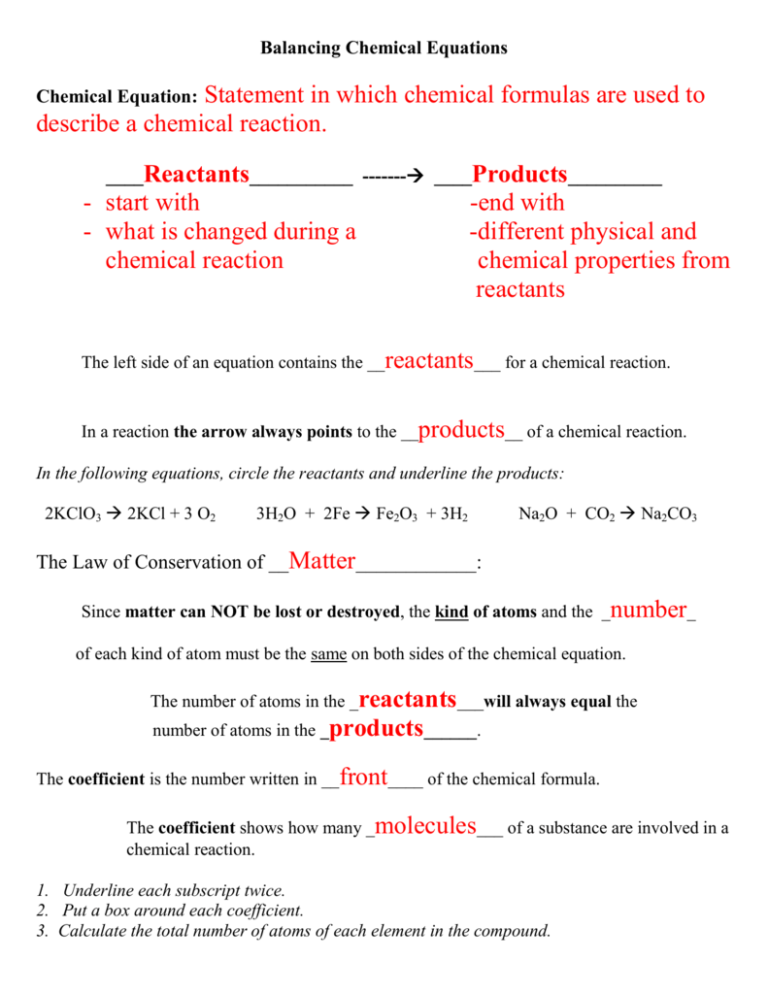
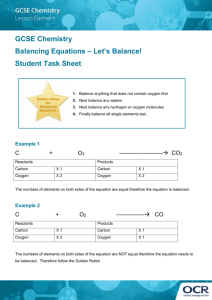
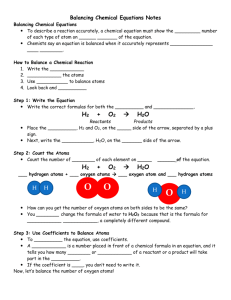
Balancing Chemical Equations Statement in which chemical formulas are used to describe a chemical reaction. Chemical Equation: ____Reactants___________ ------- ____Products__________ - start with - what is changed during a chemical reaction -end with -different physical and chemical properties from reactants The left side of an equation contains the __reactants___ for a chemical reaction. In a reaction the arrow always points to the __products__ of a chemical reaction. In the following equations, circle the reactants and underline the products: 2KClO3 2KCl + 3 O2 3H2O + 2Fe Fe2O3 + 3H2 Na2O + CO2 Na2CO3 The Law of Conservation of __Matter____________: Since matter can NOT be lost or destroyed, the kind of atoms and the _number_ of each kind of atom must be the same on both sides of the chemical equation. The number of atoms in the _reactants___will always equal the number of atoms in the _products______. The coefficient is the number written in __front____ of the chemical formula. The coefficient shows how many _molecules___ of a substance are involved in a chemical reaction. 1. Underline each subscript twice. 2. Put a box around each coefficient. 3. Calculate the total number of atoms of each element in the compound. Total number of atoms of an element = coefficient X subscript 3 Na2O 5 H2SO4 Na sodium = 3 X 2 = 6 _ H hydrogen = 5 X 2 =10__________ O = oxygen 3 X 1 = 3 S sulfur= 5 X 1 = 5 __ O oxygen = 5 X 4 = 20 _ To balance the equation only the ________Coefficient____ can be changed. 1. ______H2 +______O2 --------- ______ H2O H hydrogen 1 X 2 = 2 H hydrogen 1 X 2 = 2 O oxygen 1 X 2 = 2 O oxygen 1 X 1 = 1 2. ______Mg + ______ HCl --------- ______MgCl2 + _____H2 Mg Magnesium 1 X 1 = 1 Mg Magnesium 1 X 1 = 1 H hydrogen 1 X 1 = 1 Hydrogen = 1 X 2 = 2 Cl chlorine 1 X 1 = 1 Cl chlorine 1 X 2 = 2 3. _____H2O + _____Fe --------- _____Fe2O3 + _____H2 H hydrogen 1 X 2 = 2 H Hydrogen 1 X 2 = 2 O oxygen 1 X 1 = 1 O oxygen 1X3=3 Fe iron 1 X 1 = 1 Fe iron 1X2=2