Chem: PS D5
advertisement

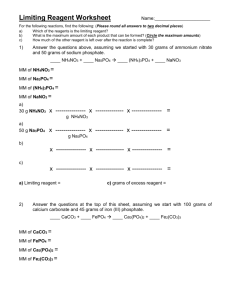
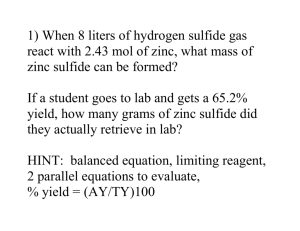
Chemistry Problem Set: D5 More Limiting Reactants/Reagents Block: ____ Name: ________________ 1) Silicon dioxide reacts with hydrogen fluoride to form silicon tetrafluoride and water. a) Write the balanced chemical reaction. b) Suppose 6.0 mol of hydrogen fluoride is added to 4.5 mol of silicon dioxide. i) What is the limiting reagent? ii) What is the excess reagent? iii) What mass (in grams) of the excess reagent remains after the reaction has completed? iv) How many grams of water are produced? 2) The mineral magnetite is actually Fe3O4. Suppose you wanted to make it by combining solid iron and water (you would need very hot iron and hot water vapor to make it happen). In addition to Fe 3O4, there would be one other product of this reaction. a) Write the balanced chemical reaction. b) Suppose you had 36 g of water and 67 g of iron. i) What is the limiting reagent? ii) What is mass of Fe3O4 produced? iii) What mass (in grams) of the excess reagent remains after the reaction has completed? 3) Ammonia and oxygen gas react to form nitrogen dioxide and water. a) Write the balanced chemical reaction. b) Suppose you had 59.5 g of ammonia and 192.0 g of oxygen gas. i) What is the limiting reagent? ii) What mass of nitrogen dioxide is produced? iii) What mass (in grams) of the excess reagent remains after the reaction has completed? 4) Metallic magnesium reacts with steam to produce magnesium hydroxide and hydrogen gas. a) Write the balanced chemical reaction. b) Suppose you had 16.2 g of metallic magnesium which is reacted with 12.0 g of water. i) What is the limiting reagent? ii) How many moles of the excess reagent remain? iii) How many grams of each product are formed? Answers (IRO) : 54 0.67 0.33 150 92.6 7.2 1.2 157.7 19.43