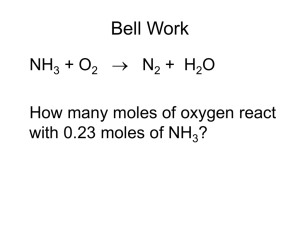Chemical Change: Stoichiometry
advertisement

Chemical Change: Stoichiometry Quantities in chemical reactions - coefficients in chemical equation give relative number of molecules, formula units or moles Mole to mole calculations - stoichiometric ratio shows molar amount of any two substances used/produced in a reaction - stoichiometric relationship expressed by general chemical reaction Calculations involving mass and moles - can find the amount of moles from mass - can then be used to find molar amounts of other substances in reaction Measuring gases in chemical change - amount of gas used/produced measured by volume (L) - ideal gas equation: PV= nRT n= PV/ RT P= pressure of gas in kPa V= volume of gas in litres n= moles of gas R= universal gas constant 8.3145 JK-1 mol-1 T= temperature of gas in Kelvin - if gas pressure is given in atm or millimitres of mercury convert it to kPa o P(kPa) = P(atm) x 101.3 o P(kPa)= P(mm Hg) x 101.3 / 760 - If temp given in Celsius convert it to Kelvin o T(K) = T(C) + 273 Stoichiometry with gas volumes - use ideal gas equation to calculate moles of gas in a reaction - can be used to find molar amounts of other reagents in equation Stoichiometry with solutions - n= cV Stoichiometry with percentage purity - many reagents are less than 100% pure - percentage purity gives percentage by mass of element/compound in impure sample - % purity= m(pure compound) x 100 / m(impure sample) Percentage yield of a chemical reaction - theoretical/maximum yield of a reaction gives amount of product that would be formed when limiting reagent is fully consumed - actual yield is usually less because o reaction may not go to completion o side reactions may give different products o may not be possible to fully extract product from final reaction mixture - % yield= actual yield x 100 / theoretical yield Limiting reagent - in a reaction one reagent is in excess, other is fully consumed (limiting reagent) - found by comparing actual mole ratio and stoichiometric mole ratio - actual ratio uses amounts given - stoichiometric ratio uses equation coefficients - if actual ratio < stoichiometric ratio then numerator is limiting reagent - if actual ratio > stoichiometric ratio then denominator is limiting reagent - ratio= n(reagent) / n(other reagent) - limiting reagent can then be used to calculate amount of any other substance in reaction