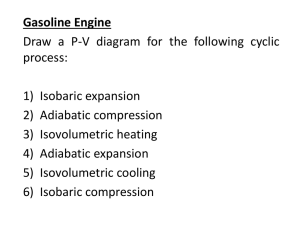
Thermodynamics Practice Test 1) If the pressure acting on an ideal
advertisement

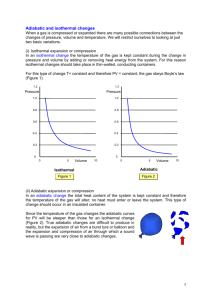
Thermodynamics Practice Test 1) If the pressure acting on an ideal gas at constant temperature is tripled, its volume is A) reduced to one-third. B) increased by a factor of three. C) increased by a factor of two. D) reduced to one-half. Answer: A 2) The number of molecules in one mole of a substance A) depends on the molecular weight of the substance. B) depends on the atomic weight of the substance. C) depends on the density of the substance. D) is the same for all substances. Answer: D 3) A container holds N molecules of an ideal gas at a given temperature. If the number of molecules in the container is increased to 2N with no change in temperature or volume, the pressure in the container A) doubles. B) remains constant. C) is cut in half. D) none of the above Answer: A 4) An aluminum rod 17.4 cm long at 20eC is heated to 100eC. What is its new length? Aluminum has a linear expansion coefficient of 25 ˛ 10-6 /Ce. A) 17.435 cm B) 17.365 cm C) 0.348 cm D) 0.0348 cm Answer: A 5) Two liters of a perfect gas are at 0eC and 1 atm. If the gas is nitrogen, N2, determine the mass of the gas. A) 2.5 g B) 2.7 g C) 2.9 g D) 3.1 g Answer: A 6) An ideal gas has a pressure of 2.5 atm, a volume of 1.0 L at a temperature of 30eC. How many molecules are there in the gas? A) 6.1 ˛ 1023 B) 6.0 ˛ 1022 C) 2.4 ˛ 1022 D) 2.3 ˛ 1023 Answer: B 7) 500 cm3 of ideal gas at 40eC and 200 kPa (absolute) is compressed to 250 cm3 and cooled to 20e C. What is the final absolute pressure? A) 748 kPa B) 374 kPa C) 200 kPa D) 100 kPa Answer: B 8) The process shown on the T-V graph is an A) adiabatic compression. B) isothermal compression. C) isochoric compression. D) isobaric compression. Answer: B 9) A gas is expanded to twice its original volume with no change in its temperature. This process is A) isothermal. B) isochoric. C) isobaric. D) adiabatic. Answer: A 10) In an isobaric process, there is no change in A) pressure. B) temperature. C) volume. D) internal energy. Answer: A 11) The process shown on the PV diagram is A) adiabatic. B) isothermal. C) isochoric. D) isobaric. Answer: C 12) Is it possible to transfer heat from a hot reservoir to a cold reservoir? A) No. B) Yes; this will happen naturally. C) Yes, but work will have to be done. D) Theoretically yes, but it hasn't been accomplished yet. Answer: B 13) During an isothermal process, 5.0 J of heat is removed from an ideal gas. What is the change in internal energy? A) zero B) 2.5 J C) 5.0 J D) 10 J Answer: A 14) The work done on an ideal gas system in an isothermal process is -400 J. What is the change in internal energy? A) zero B) -400 J C) 400 J D) none of the above Answer: A 15) A heat engine receives 7000 J of heat and loses 3000 J in each cycle. What is the efficiency? A) 57% B) 30% C) 70% D) 43% Answer: A 16) A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 ˛ 106 kg of coal each day. The heat of combustion of coal is 3.3 ˛ 107 J/kg. The steam that drives the turbines is at a temperature of 300eC, and the exhaust water is at 37eC. What is the overall efficiency of the plant for generating electric power? A) 33% B) 37% C) 46% D) 54% Answer: A 17) What is the Carnot efficiency of an engine which operates between 450 K and 310 K? A) 31% B) 41% C) 59% D) 69% Answer: A