W - Helios

Review for Final
Physics 313
Professor Lee Carkner
Lecture 25
Final Exam
Final is Tuesday, May 18, 9am
75 minutes worth of chapters 9-12
45 minutes worth of chapters 1-8
Same format as other tests (multiple choice and short answer)
Worth 20% of grade
Three formula sheets given on test (one for
Ch 9-12 and previous two)
Bring pencil and calculator
Exercise #24 Maxwell
Set escape velocity equal to maximum
Maxwell velocity
(2GM/R) ½ = 10(3kT/m) ½
m = (150KTR/GM)
Planetary atmospheres
Earth: m > 9.5X10
-27 kg (NH
3
, O
2
)
Jupiter: m > 1.4X10
-28 kg (He, NH
3
, O
2
)
Titan: m > 5.6X10
-26 kg (None)
Moon: m > 2.2X10
-25 kg (None)
Thermal Equilibrium
Two identical metal blocks, one at 100 C and one at 120 C, are placed together. Which transfers the most heat?
Two objects at different temperatures will exchange heat until they are at the same temperature
Zeroth Law: Two systems in thermal equilibrium with a third are in thermal equilibrium with each other
Heat Transfer
Heat:
Q = mc D T = mc(T f
-T i
)
Conduction: dQ/dt = -KA(dT/dx)
Radiation
Q/t = -KA(T
1
-T
2
)/x dQ/dt = A es (T env
4 -T 4 )
Temperature
How would you make a tube of mercury into a
Celsius thermometer? A Kelvin thermometer?
Thermometers defined by the triple point of water
A system at constant temperature can have a range of values for the other variables
Isotherm
Measuring Temperature
Thermometers
T (X) = 273.16 (X/X
TP
)
Temperature scales
T (R) = T (F) + 459.67
T (K) = T (C) + 273.15
T (R) = (9/5) T (K)
T (F) = (9/5) T (C) +32
Equations of State
If the temperature of an ideal gas is doubled while the volume stays the same, what happens to the pressure?
Equation of state detail how properties change with temperature
Increasing T will generally increase the force and displacement terms
Mathematical Relations
General Relations: dx = ( x/ y) z dy + ( x/ z) y dz
( x/ y) z
( x/ y) z
Specific Relations:
= 1/( y/ x)
( y/ z) x
( z/ x) z y
= -1
Volume Expansivity: b = (1/V)(dV/dT)
P
Isothermal Compressibility: k =-(1/V)(dV/dP)
T
Linear Expansivity: a = (1/L)(dL/dT)
Young’s modulus: Y = (L/A)(d t /dL)
T t
Work
How much work is done in an isobaric compression of a gas at 1 Pa from 2 to 1 m 3 ?
The work done a system is the product of a force term and a displacement term
No displacement, no work
Compression is positive, expansion is negative
Work is area under PV (or XY) curve
Work is path dependant
Calculating Work
dW = -PdV
W = PdV
For ideal gas P = nRT/V
Examples:
Isothermal ideal gas:
W = -nRT (1/V) dV = -nRT ln (V f
/V i
)
Isobaric ideal gas:
W = -P dV = -P(V f
-V i
)
First Law
Rank the following processes in order of increasing internal energy:
Adiabatic compression
Isothermal expansion
Isochoric cooling
Energy is conserved
Internal energy is a state function, work and heat are not
First Law Equations
D U = U f
-U i
= Q+W dU = dQ +dW dU = CdT - PdV
Ideal Gas
If the volume of an ideal gas is doubled and the pressure is tripled isothermally, how does the internal energy change?
lim (PV) = nRT
(dU/dP)
T
(dU/dT)
V dQ = C
V
C
P
= (dU/dV)
T
= C
V
= C
+ nR dT+PdV = C
P
V
= 0 dT-VdP
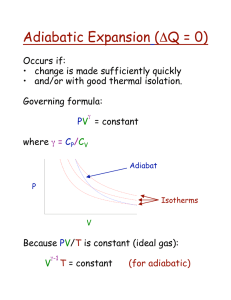
Adiabatic Processes
Can an adiabatic process keep constant
P, V, or T?
PV g = const
TV g -1 = const
T/P ( g -1)/ g = const
W = (P f
V f
- P i
V i
)/ g -1
Kinetic Theory
If the rms velocity of gas molecules doubles what happens to the temperature and internal energy
(1/2)mv 2 = (3/2)kT
U = (3/2)NkT
T = mv 2 /3k
Engines
If the heat entering an engine is doubled and the work stays the same what happens to the efficiency?
Engines are cycles
Change in internal energy is zero
Composed of 4 processes h = W/Q
H
= (Q
H
Q
H
-Q
L
)/Q
H
= W + Q
L
= 1 - Q
L
/Q
H
Types of Engines
Otto
Adiabatic, Isochoric h = 1 - (T
1
/T
2
)
Diesel
Adiabatic, isochoric, isobaric h = 1 - (1/ g )(T
4
-T
1
)/(T
3
-T
2
)
Rankine (steam)
Adiabatic, isobaric
Stirling
Isothermal, isochoric
Refrigerators
Transfer heat from low to high T with the addition of work
Operates in cycle
Transfers heat with evaporation and condensation at different pressures
K = Q
L
/W
K = Q
L
/(Q
H
-Q
L
)
Second Law
Is an ice cube melting at room temperature a reversible process?
Kelvin-Planck
Cannot convert heat completely into work
Clausius
Cannot move heat from low to high temperature without work
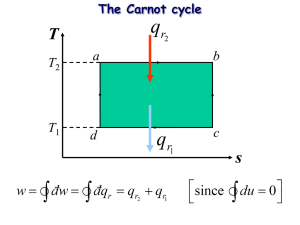
Carnot
What two processes make up a Carnot cycle? How many temperatures is heat transferred at?
Adiabatic and isothermal h = 1 - T
L
Most efficient cycle
/T
H
Efficiency depends only on the temperature
Second Law
The second law of thermodynamics can be stated:
Engine cannot turn heat completely into work
Heat cannot move from low to high temperatures without work
Efficiency cannot exceed Carnot efficiency
Entropy always increases
Entropy
Entropy change is zero for all reversible processes
All real processes are irreversible
Can compute entropy for an irreversible process by replacing it with a reversible process that achieves the same result
Entropy change of system + entropy change of surroundings = entropy change of universe
(which is > 0)
Determining Entropy
Can integrate dS to find D S dS = dQ/T
D S = dQ/T (integrated from T i
Examples: to T
Heat reservoir (or isothermal process)
D S = Q/T
Isobaric
D S = C
P ln (T f
/T i
) f
)
Pure Substances
Can plot phases and phase boundaries on a
PV, PT and PTV diagram
Saturation
condition where substance can change phase
Critical point
above which substance can only be gas
where ( d P/ d V) =0 and ( d 2 P/ d V 2 ) = 0
Triple point
where fusion, sublimation and vaporization curves intersect
Properties of Pure Substances
c c
P
V
= (dQ/dT)
= (dQ/dT)
P
(per mole)
T
(per mole) b = (1/V)(dV/dT) k = -(1/V)(dV/dP)
c
P
, c
V temperature and then level off
P
T and b are 0 at 0 K and rise sharply to the Debye
c
P and c
V end up near the Dulong and Petit value of 3R
k is constant at a finite value at low T and then increases linearly
Characteristic Functions and
Maxwell’s Relations
Legendre Transform: df = udx +vdy g= f-ux dg = -xdu+vdy
Useful theorems:
( d x/ d y) z
( d x/ d y)
( d y/ d z) x f
( d y/ d z)
( d z/ d x) y
=-1 f
( d z/ d x) f
=1 dU = -PdV +T dS dH = VdP +TdS dA = - SdT - PdV dG = V dP - S dT
( d T/ d V)
S
( d T/ d P)
( d S/ d V)
S
T
( d S/ d P)
T
= - ( d P/ d S)
V
= ( d V/ d S)
P
= ( d P/ d T)
V
= -( d V/ d T)
P
Key Equations
Entropy
T dS = C
V
T dS = C
P
Internal Energy dT + T ( d P/ d T)
V dT - T( d V/ d T)
P dV dP
( d U/ d V)
( d U/ d P)
T
Heat Capacity
T
= T ( d P/ d T)
V
= -T ( d V/ d T)
P
- P( d
- P
V/ d P)
T
C
P
- C
V
= -T( d V/ d T) c
P
- c
V
P
2 (
= Tv b 2 / k d P/ d V)
T
Joule-Thomson Expansion
Can plot on PT diagram
Isenthalpic curves show possible final states for an initial state m = (1/c
P
- v] = slope
Inversion curve separates heating and cooling region
P
)[T(dv/dT) m = 0
Total enthalpy before and after throttling is the same
For liquefaction: h i
= yh
L
+ (1-y)h f
Clausius-Clapeyron Equation
Any first order phase change obeys:
(dP/dT) = (s f -s i )/(v f - v i )
= (h f - h i )/T (v f -v i )
dP/dT is slope of phase boundary in PT diagram
Can change dP/dT to D P/ D T for small changes in P and T
Open Systems
For a steady flow open systems mass and energy are conserved:
S in
S m in
= S m out
[Q + W + m q ] = S out
[Q + W + m
Where q is energy per unit mass or: q ] q = h + ke +pe (per unit mass)
Chemical potential = m = ( d U/ d n) m i
= m f
For open systems in equilibrium: