Periodic Trends - EPIC Chemistry
advertisement

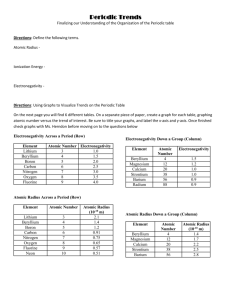
Periodic Trends Finalizing our Understanding of the Organization of the Periodic table Directions: Read the following paragraph to identify the definitions of the 3 main trends seen within the periodic table. Once you come across a definition, underline it! Once you have competed the reading, continue to answer the questions below. Reading for Information: Periodic Trends Even though it’s difficult to define the "size" of an atom, since it is too small to hold, atoms do behave in many ways as though they have characteristic sizes. The atomic radius (AR) of an atom, which is the size of the atom, ranges from element to element. A million carbon atoms placed side by side would be less than 0.2 millimeters long! Ionization energy (IE) is the energy needed to remove an electron from an atom. In other words, it is a measure of how much energy is required to remove a valence electron, and thus a measure of how tightly the atom is holding the electron. The electron configurations of the elements in group 18 are very stable because they have a full valence layer of electrons. They have a very high IE and it is extremely difficult to break an electron away, and therefore these elements with 8 valence electrons do not react with other atoms, because they do not need electrons. On the other hand, elements more willing to give up electrons to become happy have low ionization energies; it takes less work to remove those electrons from such a giving element. Electronegativity (EN) is the relative attraction of electrons in a bond. In HCl, the Cl is more electronegative than the H and attracts the electrons. The electrons spend more time around the Cl than the H because Cl has more attraction for electrons (higher electronegativity). In other words, electronegativity is the ability of an atom to attract electrons. 1. What is the definition of Atomic Radius? 2. What is the definition of Ionization Energy? 3. What is the definition of Electronegativity? 4. What elements have the greatest Ionization Energy? 5. What groups of elements do you think will have the lowest Ionization Energies? Directions: Using Graphs to Visualize Trends on the Periodic Table On the next page you will find 6 different tables. On a separate piece of paper, create a graph for each table, graphing atomic number versus the trend of interest. Be sure to title your graphs, and label the x-axis and y-axis. Once finished check graphs with Ms. Herndon before moving on to the questions below Periodic Trends Finalizing our Understanding of the Organization of the Periodic table Electronegativity Across a Period (Row) Electronegativity Down a Group (Column) Element Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Atomic Number Electronegativity 3 1.0 4 1.5 5 2.0 6 2.5 7 3.0 8 3.5 9 4.0 Element Beryllium Magnesium Calcium Strontium Barium Radium Atomic Number 4 12 20 38 56 88 Electronegativity 1.5 1.2 1.0 1.0 0.9 0.9 Atomic Radius Across a Period (Row) Element Atomic Number Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon 3 4 5 6 7 8 9 10 Atomic Radius (10-10 m) 2.1 1.4 1.2 0.91 0.75 0.65 0.57 0.51 Ionization Energy Across a Period (Row) Atomic Radius Down a Group (Column) Element Beryllium Magnesium Calcium Strontium Barium Atomic Number 4 12 20 38 56 Atomic Radius (10-10 m) 1.4 1.7 2.2 2.5 2.8 Ionization Energy Down a Group (Column) Element Atomic Number Ionization Energy Element Atomic (J/mol) Number Lithium 3 519 Beryllium 4 Beryllium 4 900 Magnesium 12 Boron 5 799 Calcium 20 Carbon 6 1088 Strontium 38 Nitrogen 7 1401 Barium 56 Oxygen 8 1036 Radium 88 Fluorine 9 1682 Neon 10 2076 Using your graphs you just drew for the above data tables, answer the following questions. 1) Does electronegativity increase or decrease left to right across a period? 2) Does electronegativity increase or decrease down a group? 3) Does ionization energy increase or decrease left to right across a period? 4) Does ionization energy increase or decrease down a group? 5) Does atomic radius increase of decrease left to right across a period? 6) Does atomic radius increase or decrease down a group? Ionization Energy (J/mol) 900 736 590 548 502 510