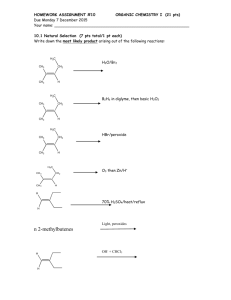
Chem 241 Lab Manual and Problem Set - 2012
advertisement

LABORATORY MANUAL ORGANIC CHEMISTRY 241 4th Edition Dr. Steven Fawl 1 LABORATORY MANUAL ORGANIC CHEMISTRY 241 FOURTH EDITION Dr. Steven Fawl Mathematics and Science Division Napa Valley College Napa, California 2 NAPA VALLEY COLLEGE COURSE: Chemistry 241 INSTRUCTOR: Dr. Steven Fawl, Room 1843, 253-3195 LECTURES: MW LABS: MT OFFICE HRS: MTW 9:30 to 10:50 2:00 to 4:50 12:20 to 1:20 Room 1843 EXAM DATES: The following are tentative dates for your exams and the material each exam will cover. Exam #1 - Wednesday, February 22nd - Spectroscopy Exam #2 - Wednesday, March 28th - Aromatics, Diels-Alder, MO Theory Exam #3 - Wednesday, May 9th - Carbonyl Chemistry Final Exam - Comprehensive - Monday, May 21st, 8:00-10:00 DESCRIPTION: A continuation of CHEM 240. Introduction to NMR, IR, and Mass Spectroscopy. Chemical reactions and syntheses of aromatic, carbonyl, and amine compounds. Special topics in carbohydrate, amino acid, and lipid chemistry. Lab work includes simple and multi-step synthesis and spectral identification. COURSE CONTENT: 1. Spectroscopy Nuclear Magnetic Resonance Infrared Spectroscopy Mass Spectrometry 2. Aromatics I: Aromaticity Reactions of benzene, Kekules structure, stability, and modern theories of the structure for benzene. Huckel's Rule and other aromatics. Nomenclature of benzene derivatives. Heterocyclic aromatics and aromatics in biochemistry. 3. Aromatics II: Reactions with Electrophiles Electrophilic aromatic substitutions and their mechanisms. Halogenation, nitration, sulfonation and alkylation of benzene. Effects of substituents-reactivity and directing influences. Alkyl and alkenyl benzenes and their reactions. Carbenes. 3 4. Aldehydes and Ketones Structure, nomenclature and physical properties. Reactions of carbonyls - acetals, hemi-acetals, ammonia derivatives, and Schiff base reactons. Keto-enol tautomerism, and the Cannizzaro Reaction. 5. Carboxylic Acids and their Derivatives Nomenclature and physical properties. Preparation and reactions at acyl carbon. Synthesis and reactions of acyl halides, acid anhydrides, esters and amides. Hell-Volhard-Zelinski and decarboxylation reactions. Claisen, Michael, and Aldol reactions and synthetic pathways. 6. Amines Nomenclature, physical properties and their basicities. Some biologically important amines. Preparation and reactions of amines with nitrous acid, diazonium salts. Analysis of amines. 7. Carbohydrates Monosaccharides, mutarotation and glycoside formation. Oxidation and reduction, osazone formation. Synthesis and degradation of monosaccharides. The D-family of aldoses. Methylation of monosaccharides. Di- and poly- saccharides. Nitrogen containing sugars. 8. Lipids Fatty acids and Triacylglycerols. Steroids and prostaglandins. Phospholipids, waxes and terpenes. 9. Amino Acids and Proteins Important amino acids. Laboratory synthesis and analysis of amino acids. Amino acid sequence of proteins and polypeptides. Primary structure of polypeptides and their synthesis. Secondary and tertiary structure of proteins. COURSE OBJECTIVES 1. 2. 3. 4. Solve complex reaction mechanisms. Synthesize compounds starting with simple ingredients. Determine the structure of organic compounds from spectrographic data. Name organic compounds based on their structure. 4 STUDENT LEARNING OUTCOMES 1. Communicate chemical and physical processes at the molecular level and how they relate to the macroscopic environment. 2. Solve synthetic reaction pathways and mechanisms while demonstrating the reasoning clearly and completely. 3. Implement laboratory techniques correctly using appropriate safety procedures and express them clearly in written laboratory reports. STUDENT ACCOMMODATION IN THE COLLEGE LEARNING ENVIRONMENT Any student who feels s/he may need accommodation based on the impact of a learning disability should contact Learning Services in the Library and Learning Resource Center (LLRC), room 1766, phone (707) 256-7442. A Learning Disability Specialist will review your needs and determine appropriate accommodations. If you need accommodations for physical or other types of disabilities, schedule an appointment with DSPS Counselor, Sheryl Fernandez, in the Counseling Department located on the top floor of the 800 building, phone (707) 253-3040 for appointment. All information and documentation is confidential. Please feel encouraged to make an appointment with me privately to discuss your specific learning needs in my class. GRADING POLICY: Three exams and a final, quizzes, plus laboratory scores will count toward the final grade according to the following schedule, 3 Exams = 300pts (100pts each) Final = 200pts Quizes = 50pts (5 @ 10pts each) Lab = 80pts Total = 630pts Grading is based on the class average = B-. The approximate breakdown of grades is, 100-85% A / 84-70% B / 69-60% C / 59-50% D / <50% F It is the policy of this class that the final exam will automatically replace the score of your lowest exam. In essence, this means that you can drop one exam and replace it with your final. There is one exception – if you are caught cheating on an exam, you will receive a zero on the exam and the final exam WILL NOT replace this grade. LABS ARE CONSIDERED LATE IF THEY ARE TURNED IN ANY TIME AFTER THE FRIDAY THAT THEY ARE DUE. LABS THAT ARE TWO WEEKS LATE WILL RECEIVE NO CREDIT. Special arrangements must be made if a lab must be missed! 5 SAFETY AND TECHNIQUE RULES Safety in the laboratory is extremely important. It is expected that you know laboratory safety rules. It is important that if you feel uncomfortable with your knowledge of these rules that you take the time to learn them. The following list is NOT complete. The Media Center, room 1028, there is a video tape available in which a Napa Valley College instructor explains in detail safety and technique rules. There is NO excuse for not following safety rules. 1) Be attentive to instructions and follow them carefully. Read the board at the back of the class room when you first come to class, any changes in procedure will be written there. 2) If you ever have any questions about the procedure, apparatus, or chemicals it is important that you ask the Instructor or Instructional assistant. 3) Do not perform any unauthorized experiments. Anyone found doing so faces permanent expulsion from class. 4) Do not handle chemicals or materials not assigned to you or not called for in the experiment. 5) Learn the location and proper use of the fire extinguisher, safety shower, eye and face wash. Keep the first aide sink area clear at all times 6) Coats, books, etc, should be kept in the space provided for them at the back of the lab. Many of the chemicals used in the lab can ruin or stain paper and clothing. 7) Never taste chemicals, nor pipet by mouth. Always use pipet bulbs or wheels. 8) Smell chemicals by fanning a little vapor towards you. 9) Experiments in which dangerous or obnoxious fumes are produced must be done in the fume hood. Be sure to stop these reactions as soon as possible. 10) No eating, drinking or smoking in the lab. 11) Never point test tubes at yourself or others. 12) In the event of any injury, spill or glass breakage inform the Instructor immediately. 13) Goggles must be worn at all times when in the lab. 14) Chemicals may not be taken out of the lab (not even to the I.A.'s desk.) 15) Chemicals may not be stored in lockers. 16) Avoid unnecessary contact with ALL chemicals. 17) Do not leave lit burners unattended. 6 18) Every time you use a chemical read it's label carefully. If any discrepancies inform the IA or instructor immediately. 19) All containers which contain a chemical or in which a reaction occurs must be labeled. 20) When labeling a storage container include name and/or formula of chemical, any appropriate warnings, concentration, date and your name. 21) NEVER place anything inside a reagent bottle, no spatulas, droppers, nor pipets. If the reagent is a clumpy solid inform the IA. Proper technique is to "roll" containers from side to side to remove solids and to pour liquids into smaller containers (such as a beaker) first. 22) NEVER return unused chemical (liquids or solids) back to the original container - offer excess to another student or dispose of it appropriately. 23) Be conservative of reagents, place only the amount you need into a labeled container (such as a beaker). Do not take the reagent bottles to your work area - leave them where every one can find them. 24) Use tap water to wash glassware - you should rinse with DI water - please be conservative. 25) To dilute acids and bases, Add the Acid (or Base) to the Water. 26) Dispose of liquids and solids appropriately, read the board, or your experimental procedure for special instructions, otherwise dispose of liquids and soluble solids down the sink with lots of water, insoluble materials (such as paper towels) should be put in the waste basket. KEEP THE SINKS CLEAN 27) It is very important to keep the lab clean. Before you leave each time be sure to: a) return equipment to its proper place b) clean up your workspace with the sponge c) put away your labware d) lock your locker There is NO reason for a messy lab. Everything you need to keep your lab neat and clean is available. Dirty counters, paper towels left in the sink or troughs, labware left out, messes left under the fume hood, chemical spills left on the balance, are BAD technique and as such will not be tolerated. 28) You may not be in the laboratory at any time other than your scheduled laboratory period unless you have the permission of the instructor in charge as well as your course instructor. Do not visit friends during their lab time and do not invite your friends or family to visit you. 7 TABLE OF CONTENTS EXPERIMENT 1 Synthesis of p-Nitroacetanilide 10 WORKSHEET 14 EXPERIMENT 2 Synthesis of Aspirin - Ester Formation 16 WORKSHEET 19 EXPERIMENT 3 Reactions of Aromatic Aldehydes - Cannizaro Reaction WORKSHEET 21 25 EXPERIMENT 4 Synthesis of 9,10-Dihyroanthracene-9,10-endo-α,β-succinic Anhydride: A Diels-Alder Reaction WORKSHEET 26 27 EXPERIMENT 5 sec-Butylbenzene - Friedel Craft Alkylation WORKSHEET 28 29 EXPERIMENT 6 Sodium Borohydride Reduction of Acetophenone WORKSHEET 30 33 EXPERIMENT 7 Synthesis of 2-Methyl-2-Hexanol: A Grignard Reaction 34 EXPERIMENT 8 Biodiesel Synthesis 42 8 EXPERIMENT ONE SYNTHESIS OF P-NITROACETANILIDE O H C O CH3 H N C CH3 N HNO3 H2SO4 Discussion: NO2 The aromatic nitration of acetanilide is an exothermic reaction ; the temperature must be carefully controlled by chilling, stirring, and the slow addition of reagents. Acetanilide is first dissolved in the solvent, glacial acetic acid, by warming. Glacial acetic acid is used because it is a polar solvent capable of dissolving acetanilide and the acetate ion is a poor nucleophile so no substitution is possible. After the solution is cooled, sulfuric acid is added; however, even with cooling, the temperature of the solution rises almost 40̊. Both the acetanilide solution and the nitrating solution (a mixture of HNO3, and H2SO4) must be chilled to about 10̊C before the reaction is begun. To prevent dinitration of the acetanilide, the nitrating mixture is added in small portions to the acetanilide solution (and not vice versa) so that the concentration of HNO3, is kept at a minimum. After all the HNO3, H2SO4 solution has been added, the reaction mixture is allowed to warm slowly to room temperature. If the reaction mixture has been kept excessively cold during the addition, there will be a relatively large amount of unreacted HNO3, present, which may cause the temperature to rise above room temperature. If this should happen, the mixture must be rechilled. The work-up procedure consists of removal of the acids and crystallization of the product. Every trace of acid must be removed because hydrogen ions catalyze the hydrolysis of the amide to p-nitroaniline or its protonated cation. Most of the acid is removed by pouring the reaction mixture onto ice and water, then filtering the flocculent yellow precipitate of p-nitroacetanilide. The last traces of acetic acid are removed by neutralization. Because bases also catalyze the hydrolysis of amides, the neutralizing agent used is disodium hydrogen phosphate (Na2HPO4). This reagent reacts with acids to yield NaH2PO4. The result is a buffered solution with a pH near neutral. The crude product is air-dried before crystallization. If all of the acid was removed, the product will be light yellow. A deep yellow to yellow-orange product is indicative of the presence of pnitroaniline from hydrolysis. Unfortunately, p-nitroaniline is difficult to remove from pnitroacetanilide by crystallization. Equipment 250-mL beaker dropper or disposable pipet 9 two 50-mL and one 125-mL Erlenmeyer flasks 10-mL graduated cylinder hot plate ice bath spatula stirring rod thermometer vacuum filtration assembly watch glass acetanilide, 6.5 g disodium hydrogen phosphate, 15 g 95% ethanol, 60 mL glacial acetic acid, 10 mL conc. nitric acid, 3.5 mL conc. sulfuric acid, 15 mL Time Required: about two hours to crude product; 15-20 minutes for crystallization; two overnight dryings; 15 minutes for melting-point determination STOPPING POINTS: during either of the two drying periods or while the product is crystallizing from ethanol >>>>SAFETY NOTE 1: A mixture of concentrated nitric and sulfuric acids is used as the nitrating mixture. Use extreme caution when preparing and using this mixture. >>>>SAFETY NOTE 2: Nitro compounds are toxic and can be absorbed through the skin. You may wish to wear disposable plastic gloves during portions of this experiment. PROCEDURE Place 6.5 g of acetanilide in a 125-mL Erlenmeyer flask, add 10 mL of glacial acetic acid (CAUTION: strong irritant), and warm the flask on a hot plate in a fume hood until the acetanilide dissolves. Cool the flask in an ice bath to about 20̊; then add 10 mL of cold, conc. sulfuric acid. The temperature of the mixture will rise to about 60̊. Chill the solution to about 10̊ in an ice bath. (The solution will become very viscous.) Mix 3.5 mL of conc. nitric acid and 5 mL of conc. sulfuric acid in a 50-mL flask, and chill the flask in an ice bath. When both solutions are cold, slowly add the HNO3, H2SO4 solution, 1 mL at a time, to the acetanilide solution. Keep the reaction flask in an ice bath so that the temperature of the reaction mixture is maintained between 10-20̊. Stir the reaction mixture carefully after each addition. The entire addition requires about 15 minutes. After the addition is completed, allow the reaction flask to stand at room temperature for 30 minutes. Monitor the temperature; if it rises above 25̊, chill the flask in an ice bath. Should the rechilling be necessary, allow the flask to stand for 30 minutes or more at room temperature after the rechilling. Pour the reaction mixture into a 250-mL beaker containing 100 mL of water and 25 g of cracked ice. Using a large Buchner funnel, filter the heavy lemon-yellow precipitate with vacuum. Press out as 10 much aqueous acid from the filter cake as possible with a spatula or clean cork while suction is being applied (CAUTION: see Safety Note 2). The precipitate is voluminous; use care in transferring it to the Buchner funnel or a substantial amount of product will be lost. Transfer the filter cake to a clean 250-mL beaker, and add 100 mL of 15% aqueous disodium hydrogen phosphate. Stir the mixture to a paste-like consistency and refilter using vacuum. Wash the beaker with two 30-mL portions of cold water. Finally, wash the filter cake with an additional 50 mL of cold water. Press the filter cake with a spatula or clean cork to remove as much water as possible, then dry the solid overnight on a watch glass. Determine the yield and melting point. The crude product can be purified by crystallization from 30-60 mL of 95% ethanol. (The crude product dissolves very slowly, even with heating; avoid using an excess of solvent.) 11 NAME DATE ________________ SYNTHESIS OF P-NITROACETANILIDE WORKSHEET Mass of Product Melting Point CRC Melting Point Theoretical Yield (grams) Actual Yield (Percent) Problems 1. List at least two reasons for the choice of glacial acetic acid as the solvent for the nitration of acetanilide. 2. What would be the effects of each of the following changes of reaction conditions in this experiment, assuming that all other conditions are held constant? Some of these are simple dilutions while others are addition of extra reactants. Explain your answers. (a) increasing the amount of glacial acetic acid from 10 mL to 20 mL (b) increasing the amount of nitric acid from 3.5 mL to 7.0 mL (c) decreasing the amount of sulfuric acid from 15 mL to 5 mL 3. Write equations for the hydrolysis of p-nitroacetanilide in (a) aqueous acid; (b) aqueous hydroxide. The products of the reation should be p-nitroaniline and acetic acid (or acetate ion in base). 4. Predict what would happen during the crystallization of p-nitroacetanilide from 95% ethanol if all the acidic material had not been neutralized previously? Use an equation in your answer. You should ask yourself, “What is the other 5% and what will it do to this reaction?” 12 EXPERIMENT TWO SYNTHESIS OF ASPIRIN: ESTER FORMATION O OH O C OH O O CH 3 C O C CH 3 OH C O O C CH3 H2SO4 Salicylic Acid Aspirin Discussion Salicylic acid is found in the bark of willow trees (trees of the genus Salix, from whence salicylic acid derives its name). Today all aspirin sold is synthesized from phenol, which, in turn, is obtained from petroleum. The last step of the commercial synthesis is the conversion of salicylic acid to aspirin using the Fisher ester synthesis. The conversion of salicylic acid to aspirin is not required in order for aspirin to work but the conversion to aspirin does make it more easily tolerated by the stomach. The reaction of salicylic acid with acetic anhydride occurs rapidly. The reactants and a sulfuric acid catalyst are mixed, then warmed in a hot water bath. Solid salicylic acid is insoluble in acetic anhydride. Aspirin is soluble in a hot mixture of acetic anhydride and acetic acid, which is formed as the reaction proceeds. Thus, the course of the reaction can be followed by the disappearance of the solid salicylic acid. The reaction is essentially complete when all the salicylic acid has dissolved. Aspirin is only slightly soluble in a cold mixture of acetic anhydride and acetic acid. Therefore, as the mixture is cooled to room temperature, the aspirin precipitates. At the end of the reaction period, the mixture contains aspirin, acetic anhydride, and acetic acid. Acetic acid is miscible with water, and acetic anhydride reacts fairly rapidly with water to yield acetic acid. By adding water to the reaction mixture and allowing the aqueous mixture to stand at room temperature for a few minutes, we can achieve a reasonable separation-the contaminants dissolve in the water, and the aspirin precipitates. Because of the presence of acetic acid and because aspirin is slightly soluble in water, about 10% of the aspirin remains in solution. Therefore, the mixture is thoroughly chilled before filtration and crystallization. 13 An additional small amount of aspirin can be recovered from the mother liquor if it is allowed to stand overnight. Despite this fact, it is not good practice to leave the bulk of the aspirin in acidic solution for an extended period of time because it will undergo a slow hydrolysis to yield salicylic acid and acetic acid, a typical acid-catalyzed ester hydrolysis. EQUIPMENT 400-mL beaker (for hot water bath) dropper two 125-mL Erlenmeyer flasks 10-mL and 100-mL graduated cylinders spatula thermometer vacuum filtration assembly two watch glasses CHEMICALS acetic anhydride, 5.0 mL salicylic acid, 2.8 g conc. sulfuric acid, 3-4 drops TIME REQUIRED: 1 hour plus overnight drying and time for a melting-point determination STOPPING POINTS: after the initial vacuum filtration; while the product is crystallizing from water; while the final product is being air-dried >> SAFETY NOTE: Acetic anhydride is volatile and is a strong irritant. PROCEDURE Place 2.8 g of salicylic acid in a dry 125-mL Erlenmeyer flask, then add 5.0 mL acetic anhydride and 3-4 drops concentrated sulfuric acid. Mix the resultant white slurry thoroughly with a spatula, and place the flask in a warm water bath (45-50̊C) for 5-7 min. Swirl or stir the mixture occasionally to dissolve all the solid material. Because the reaction is slightly exothermic, a small temperature rise can be detected. Allow the flask to cool. The aspirin begins to precipitate when the temperature of the solution is about 35-40̊C, and the mixture becomes semisolid. When this occurs, add 50 mL water and break up any lumps with a spatula. Allow the mixture to stand for an additional 5 minutes, then chill the flask in an ice bath and remove the crystals by vacuum filtration. Crystallize the crude aspirin from 25 mL of warm water not exceeding 80̊C (see Experimental Note). Allowing the mother liquor to sit overnight may produce a second crop of crystals. Air-dry the crystals and determine the percent yield and melting point. 14 EXPERIMENTAL NOTE At temperatures exceeding 80̊C, aspirin forms an oil that dissolves organic impurities from the water; in this case, it may be difficult to redissolve the aspirin in water. If the solid does not dissolve in 25 mL of water, add more water from a dropper. Let the mixture warm 2-4 minutes between additions to allow the solid to dissolve. 15 NAME DATE ________________ SYNTHESIS OF ASPIRIN WORKSHEET Mass of Product Melting Point CRC Melting Point Theoretical Yield (grams) Actual Yield (Percent) PROBLEMS 1) Write an equation for the synthesis of aspirin from salicylic acid with an acid chloride instead of acetic anhydride. 2) The following compounds all have antipyretic and analgesic properties similar to aspirin. Suggest a synthesis for each from salicylic acid. (a) sodium salicylate (b) methyl salicylate (c) salicylamide (d) phenyl salicylate (e) 2-0-acetyl-5-bromosalicylic acid 16 3a) Commercial aspirin sometimes has a distinct acetic acid odor. Why? 3b) Would ingestion of such aspirin be harmful? Explain. 17 EXPERIMENT THREE THE CANNIZARO REACTION: THE DISPROPORTIONATION OF BENZALDEHYDE H H O HO C DISCUSSION O H C OH C KOH + 2x In planning the laboratory schedule, it should be observed that this experiment requires materials to be mixed and allowed to stand for 24 hr or longer. In the presence of strong alkalis, benzaldehyde (like formaldehyde) undergoes disproportionation to form the corresponding primary alcohol and a salt of the carboxylic acid : the Cannizzaro reaction. The process involves addition of hydroxyl ion to the carbonyl group of one molecule and transfer of hydride anion from the adduct to a second molecule of benzaldehyde, accompanied by proton interchange to form the benzoate anion and benzyl alcohol. If the reaction is effected under anhydrous conditions with the sodium derivative of benzyl alcohol as catalyst, the product is the ester, benzyl benzoate. EQUIPMENT 18 g KOH 20 mL Benzaldehyde 125 mL Erlenmeyer/stopper 100 mL methylene chloride Steam bath 125 mL separatory funnel 10 mL 20% sodium bisulfite 4 g anhydrous magnesium sulfate 100 mL round bottom flask Thermometer (0-250̊C) Bunsen burner 40 mL conc. HCl Chipped ice Blue litmus paper Side arm flask Buchner funnel EXPERIMENT: In a small beaker dissolve 0.27 mole (18 g of 85% pure solid) of solid potassium hydroxide in 18 mL of water and cool the solution to about 25̊C. Place 0.2 mole (21 g, 20 mL) of benzaldehyde in a 125-mL Erlenmeyer flask (or narrow-mouth bottle) and to it add the potassium hydroxide solution. Cork the flask firmly and shake the mixture thoroughly until an emulsion is formed. Allow the mixture to stand for 24 hr or longer. At the end of this period, the odor of benzaldehyde should no longer be detectable. 18 Isolation of Benzyl Alcohol To the mixture add just enough distilled water to dissolve the precipitate of potassium benzoate. Shake the mixture thoroughly to facilitate solution of the precipitate. Extract the alkaline solution with three or four 20-mL portions of methylene chloride to remove the benzyl alcohol and traces of any unconverted benzaldehyde. Combine the methylene chloride extracts for isolation of benzyl alcohol and reserve the aqueous solution to obtain the benzoic acid. Concentrate the methylene chloride solution of benzyl alcohol by distillation on a steam bath using a water-cooled condenser, until the volume of the residual liquid has been reduced to 15-20 mL. A steam bath is just a beaker of boiling water into which the distillation flask is emersed. Cool the remaining liquid, transfer it to a small separatory funnel (using 2-3 mL of methylene chloride to rinse the distilling flask), and shake it thoroughly with two 5-mL portions of 20% aqueous sodium bisulfite to remove any benzaldehyde. Wash the methylene chloride solution finally with two 10-mL portions of water and dry it with 3-4 g of anhydrous magnesium sulfate. Filter the solution into a small dry distilling flask and carefully distill off the methylene chloride. Attach a short air-cooled condenser and distill the benzyl alcohol, by heating the flask directly with a luminous flame kept in motion. Collect the material boiling at 200-206̊C. The yield is 4-5 g. Isolation of Benzoic Acid To free the acid, pour the aqueous solution of potassium benzoate (from which the benzyl alcohol has been extracted) into a vigorously stirred mixture of 40 mL of concentrated hydrochloric acid, 40 mL of water, and 40-50 g of chipped ice. Test the mixture with indicator paper to make sure that it is strongly acidic. Collect the benzoic acid with suction and wash it once with cold water. Crystallize the product from hot water, collect the crystals, and allow them to dry thoroughly. The yield is about 8g. I.R. SPECTROSCOPY Setup Instructions 1. Remove the cover from the machine. 2. The "ON" switch is located to the rear on the right side of the machine. Switch the machine on. The spectrometer will take about a minute to warm-up. When it is ready for use it will beep twice. 3. Running the machine involves several simple steps. The machine can scan at two rates, a three minute and a twelve minute scan. A three minute scan will give you all of the major spectroscopic peaks, but none of the details. The twelve minute scan will give you the details that the three minute scan missed. Set the machine to a three minute scan. 19 4. There are several kinds of chart paper that can be used for plotting the output. We use the shortest sheets. Set the machine for short paper output. 5. Before a plot can be made a pen must be placed into the slide bar located near the center of the machine. The pens are located on a shelf next to the spectrometer (several colors are available). Slide one of the pens (any color) into the pen holder on the slide bar. 6. Occasionally the edge of the chart paper becomes misaligned with the pen position. The chart can be moved by pressing the Chart button (either the UP or the DOWN button) to move the chart into the proper position. The spectrometer is now ready to accept a sample. Sample Preparation Infrared spectroscopy can be done on either liquid or solid samples. The preparation of these samples differ dramatically. Follow these general guidelines. Liquid Samples Liquid samples are loaded into "cells". What this means is that the liquid will be sandwiched between two plates. Each plate has a shallow indentation in it's surface that holds a small amount of sample. When the plates are put together the sample is trapped in this indentation producing a thin film of sample which can be analyzed by the spectrophotometer. The process is very simple. Locate the cell and it's holder. It should be found in a small white box on a shelf adjacent to the machine. The cell holder is a round piece of white plastic with a hole in the center and should be found together with small piece of protective cloth which contains the cells themselves. The cells are small round disks of what appear to be plastic but they are really made of solid AgCl so be careful with them. You will notice that the cell holder unscrews. Unscrew the cell holder and inside you will see a black rubber "O" ring. Take one of the two cells and place it, shallow side up, on top of the "O" ring. Place two or three drops of sample onto the cell surface and quick place the other half cell and place it on top (shallow side down). Now screw the top of the cell holder back onto the cell. The cell should be snug but not overly tight. Overtightening can warp or even break the AgCl cells. Please be careful. Now that the sample is in the cell you are ready to mount the cell into the spectrometer and take a spectrum of the sample. Solid Samples Solid samples can be prepared by mixing (actually grinding) the solid together with KBr. You will do this using an agate morter and pestle. It is usually wise to use about two to three times more KBr than the amount of solid sample (eye-ball it). Use very small amounts of each, 1 gram of KBr and 0.3 grams of solid are more than enough (usually). After the sample has been well mixed place a small 20 amount in the KBr wafer press and make a thin, nearly transparent wafer of this mixture. Small cracks in the sample are alright. Mount the pellet in the IR and run the sample. Problem Write equations for the preparation of benzaldehyde from (a) benzene (b) toluene (c) benzoic acid 21 NAME DATE ________________ REACTIONS OF AROMATIC ALDEHYDES WORKSHEET Results: Benzyl Alcohol Mass of Benzyl Alcohol Ref. Index of Benzyl Alcohol Boiling Point of Benzyl Alcohol Theoretical Yield (grams) Actual Yield (Percent) Results: Benzoic Acid Mass of Benzoic Acid Melting Point of Benzoid Acid Theoretical Yield (grams) Actual Yield (Percent) Attach your spectra to this sheet and locate the following peaks by circling and labeling them. Benzyl Alcohol OH stretch CH stretch Benzene C=C stretch C-O stretch Benzoic Acid OH stretch CH stretch Benzene C=C stretch C-O stretch C=O stretch 22 EXPERIMENT FOUR Synthesis of 9,10-Dihyroanthracene-9,10-endo-α,β-succinic Anhydride A Diels-Alder Reaction O O O + O O O EQUIPMENT 2.0 g Anthracene 50 mL round bottom flask 55 mL mixed xylenes 1.0 g maleic anhydride Reflux condenser Ice bath Buchner funnel Side-arm flask 10 mL iced xylene Watch glass Parafilm EXPERMENT Place 2.0 g of anthracene in a 50 mL round bottom flask and add 25 mL of mixed xylenes. Add 1.0 g of well ground maleic anhydride, fit the flask with a reflux condenser, and heat the mixture at reflux for 30 minutes. Allow the flask to cool to room temperature, then chill it in an ice bath. The product if fairly insoluble in xylene. Once crystallization begins, it cannot be reversed by heating the solution. Filter the solid using a Buchner funnel and wash the product with 10 mL of of ice-cold mixed xylene. Dry the solid on a watch glass next to a small beaker of paraffin wax, both covered by a larger beaker. A typical yield is 2.5 g of colorless product mp 262-264̊C. If the sample is tinged with yellow and has a depressed melting point, recrystallize the product from xylene (20 mL per gram of product). 23 NAME DATE ________________ Synthesis of 9,10-Dihyroanthracene-9,10-endo-α,β-succinic Anhydride A Diels-Alder Reaction Worksheet Mass of Product Melting Point CRC Melting Point Theoretical Yield (grams) Actual Yield (Percent) Problems 1) Predict the products of hydrolysis (cleavage by water) of the following anhydrides. O CH3 CH2 O C O C CH3 O O O 2) What products would be obtained from a Diels-Alder reaction of anthracene with following dienophiles? A. CH3 C H O C C O C6 H 5 H O B. C. H C C C O CH3 O 24 EXPERIMENT FIVE Friedel Craft Alkylation: Formation of sec-Butylbenzene H3 C C C CH3 C-C-C-C-Br DISCUSSION AlCl3 The reaction between bromobutane and benzene in the presence of AlCl3 is a classic Friedel-Craft alkylation reaction. The reaction begins with the removal of the bromine by the AlCl3 to produce a bromobutane carbocation. This cation rearranges to place the positive charge on the secondary carbon of butane. Thus, when benzene attacks the butane carbocation the attachment is on the secondary rather than the primary carbon. The only product formed in this reaction is sec-butylbenzene. No butylbenzene is formed. EQUIPMENT 250 mL round bottom flask condenser 7.8 mL n-butyl bromide 50 mL benzene 3.25 g Anhydrous Aluminum chloride 6 mL conc. HCl 40 g ice steam bath 250 mL separatory funnel 3-4 g CaCl2 thermometer (0-200̊C) EXPERIMENT In a dry 250 mL round-bottomed flask fitted with an upright condenser, place 11g (7.8 mL) of n-butyl bromide and 50 mL of benzene. To provide for the acid vapors evolved during the reaction do the experiment in the hood. Add 0.025 mole (3.25g) of pulverized anhydrous aluminum chloride and allow the reaction to proceed in the cold, shaking the flask occasionally on a steam bath, and finally reflux the mixture on a steam bath for about an hour. Cool the reaction mixture and pour it with stirring into a mixture of 40 g ice, 25 g water, and 6 mL of concentrated HCl. Stir thoroughly to dissolve the excess aluminum compounds and transfer the mixture to a large separatory funnel. Separate the benzene layer and dry it with 3-4 g anhydrous calcium chloride, and decant the dried liquid into a 250 mL round bottom flask. Fit the flask with a fractionating column and distill the mixture. Distill very slowly but at a regular rate. Save all the sample that distills above 130̊C and put this into a 50 mL round bottom flask and refractionate, keeping the ethylbenzene fraction which boils from 173-178̊C. 25 NAME DATE ________________ sec-Butyl Benzene Worksheet Mass of Product Boiling Point CRC Boiling Point Theoretical Yield (grams) Actual Yield (Percent) Questions 1) Why is such a large excess of benzene used in this experiment? 2) What products would be formed by the reaction of the following alkenes with benzene, in the presence of aluminum chloride: ethylene? isobutylene? cyclohexene? 3) Explain why n-propyl bromide reacts with benzene in the presence of aluminum chloride to form mainly isopropylbenzene. How could you make propylbenzene? 4) Write the reactions for the formation of; diphenylmethane triphenylmethane p-chloroethylbenzene 26 EXPERIMENT SIX SODIUM BOROHYDRIDE REDUCTION OF ACETOPHENONE O CH3 HO CH3 NaBH4 DISCUSSION The reduction of an aldehyde or ketone with sodium borohydride is straight forward and usually affords a high yield of the alcohol. The usual procedure (and the one employed in this experiment) involves dissolving the borohydride in 95% ethanol and adding the carbonyl compound to this solution. To ensure complete reaction, an excess of sodium borohydride is used. The reaction between sodium borohydride and acetophenone is exothermic. Therefore, it is important to add the acetophenone drop-wise and to control the reaction temperature with an ice bath. After the reaction has been completed, the excess borohydride and the ethoxyborohydrides are destroyed with aqueous acid. Because hydrogen gas is evolved, this treatment with acid must be carried out in a fume hood or a very well-ventilated room. Because ethanol, the reaction solvent, is water-soluble, a clean separation of organic and inorganic products cannot be achieved by a simple extraction with water and diethyl ether at this point. (Too much product would be lost in the aqueous ethanol layer.) To circumvent this problem, the first step in the work-up is to boil off much of the ethanol. In a larger-scale reaction, the ethanol would be distilled and collected. In a small-scale reaction such as in this experiment, the ethanol can be boiled away in the fume hood. When most of the ethanol has been removed, the product 1-phenylethanol oils out. Water and diethyl ether are then added to the residue for the extraction of the organic compounds from the inorganic salts. The ether extract is dried with either sodium sulfate or magnesium sulfate. The crude product is obtained by distilling the ether. Because of its high boiling point, 1-phenylethanol cannot be distilled at atmospheric pressure. Although it could be vacuum-distilled, distillation could not separate it from unreacted starting material (if any) because the two compounds have boiling points only 1̊ apart. (An infrared spectrum can be used to determine if any ketone is present in the product.) 27 EQUIPMENT 150-mL beaker distillation apparatus dropper three 50-mL Erlenmeyer flasks 10-mL and 50-mL graduated cylinders hot plate or steam bath with magnetic stirrer (optional) ice bath two 50-mL round-bottom flasks 125-mL separatory funnel thermometer CHEMICALS acetophenone, 12.0 g anhydrous magnesium sulfate (or Na2SO4) (1 g) diethyl ether, 40 mL 95% ethanol, 30 mL 3M hydrochloric acid, 10 mL sodium borohydride, 1.5 g TIME REQUIRED: about 4 hours STOPPING POINTS: after the acetophenone has been added; after the excess ethanol has been boiled off ; while the ether solution is drying >>>> SAFETY NOTE: Sodium borohydride is caustic. Do not let it come into contact with your skin. If accidental contact should occur, wash immediately and thoroughly. PROCEDURE: Place 1.5 g of sodium borohydride (see Safety Note) in a 150-mL beaker. Add 30 mL of 95% ethanol and stir until the solid is dissolved (see Experimental Note). Weigh 12.0 g of acetophenone into a 50mL Erlenmeyer flask, and prepare an ice bath. Add the acetophenone dropwise to the borohydride solution while stirring the mixture continuously, preferably with a magnetic stirrer. Keep the temperature of the reaction mixture between 30-50̊C by controlling the rate of addition and by cooling the beaker in the ice bath as necessary. As the acetophenone is added, a white precipitate forms. The addition should take about 45 minutes. After the addition is complete, allow the reaction mixture to stand at room temperature for 15 minutes with occasional stirring (or continuous stirring if you are using a magnetic stirrer). (Stopping Point) In the fume hood, add about 10 mL of 3M HCl to the reaction mixture. After the reaction has subsided, heat the mixture to boiling on a hot plate or steam bath in the fume hood until the mixture separates into two layers. 28 (Stopping Point) Cool the reaction mixture in an ice bath, then transfer it to a separatory funnel. Wash the residual material in the beaker into the separatory funnel with 20 mL of diethyl ether (flammable). If the inorganic salts precipitate, add 20-40 mL of water, as necessary, to dissolve them. Extract the aqueous layer with this ether, then with a second 20-mL portion of ether; combine the ether extracts; wash them with an equal volume of water; and dry them with anhydrous magnesium sulfate or sodium sulfate. (Stopping Point) Decant or filter the dried solution into a tared flask, and distil the ether slowly, using a heating mantle or steam bath and an efficient condenser. Do not overheat the flask. You are not keeping the ether that is being removed, keep what is left in the tared flask. A typical crude yield is 10 g (82%). Measure the refractive index, and run the infrared spectrum (thin film). From these two pieces of data, estimate the purity of the crude product. EXPERIMENTAL NOTE Fresh sodium borohydride dissolves in 95% ethanol, but partially hydrolyzed sodium borohydride will not all dissolve. This should not affect the experiment because an excess of borohydride is used. (If your borohydride is extremely poor quality, your instructor may suggest that you use a greater excess than is called for.) 29 Name DATE ________________ SODIUM BOROHYDRIDE REDUCTION WORKSHEET Mass of Product Ref. Index CRC Ref. Index Theoretical Yield (grams) Actual Yield (Percent) Attach the I.R. Spectrum of the Product Locate the position of the following peaks in your spectrum; -OH peak -phenyl peak (benzene stretch) -CH stretch -C-OH stretch PROBLEMS 1) Write an equation for the hydrolysis of NaBH4. The products should be NaH2BO3 and H2 gas. 2) Under what circumstances would you expect to find unreacted acetophenone in the product in this experiment? 3) Which compound would you expect to undergo borohydride reduction more rapidly? Explain. (a) CH3CH2CHO or (b) CH3CH2COCH2CH3 30 EXPERIMENT SEVEN SYNTHESIS OF 2-METHYL-2-HEXANOL: A GRIGNARD REACTION DISCUSSION A standard Grignard synthesis is carried out in three steps: (1) preparation of RMgX; (2) the reaction of RMgX with the carbonyl compound or other reactant; and (3) the acidic hydrolysis. The first two steps (and often all three steps) are generally carried out in the same reaction vessel. The intermediate products (the Grignard reagent,and the alkoxide) are rarely isolated. PREPARATION OF THE GRIGNARD REAGENT (STEP 1) In this experiment, the Grignard reagent is prepared by slowly adding a solution of 1-bromobutane in anhydrous diethyl ether (not solvent ether, which is wet) to Mg turnings. Unfortunately, the reaction leading to the formation of a Grignard reagent is often difficult to initiate. Difficulties can usually be traced to contaminants, primarily water. Therefore, scrupulous care must be taken to dry all glassware and to use only dry reagents and solvents. The techniques used to start the reaction are discussed in Experimental Note 3. Once started, the formation of a Grignard reagent is exothermic; therefore, excess 1-bromobutane should not be added to initiate the reaction. If a large excess of 1bromobutane is present in the reaction vessel, the reaction may be difficult to control. Once the reaction has begun, the 1-bromobutane is added at a rate that will maintain a gentle reflux of the ether in the reaction flask. At the end of the reaction, some of the magnesium may remain unconsumed. The reason for this is that some 1bromobutane is destroyed by undergoing a coupling reaction with the Grignard reagent to yield octane. Other than providing a mechanical inconvenience in the extraction steps, the residual magnesium metal does not interfere with the remainder of the experiment. An ether solution of a Grignard reagent has a translucent gray-to-black tint. The color arises from impurities in the magnesium metal, rather than from the Grignard reagent itself. Once formed, the Grignard reagent must be carried on to Step 2 (the reaction with acetone) immediately. It cannot be saved until the next laboratory period because it reacts with oxygen and moisture from the air. 1: Grignard apparatus 31 REACTION WITH ACETONE (STEP 2) The reaction of n-butylmagnesium bromide with acetone is extremely vigorous. The acetone must be added very slowly; otherwise, the reaction mixture will boil over. The product is a magnesium alkoxide of an alcohol and thus insoluble in diethyl ether. This alkoxide sometimes forms a crusty precipitate that must be broken up by swirling the flask so that the unreacted acetone can become mixed with the Grignard reagent. After the reaction of acetone and the Grignard reagent is completed, it is no longer necessary to protect the reaction mixture from air or moisture. This mixture can be stored until the next laboratory period. HYDROLYSIS OF THE ALKOXIDE (STEP 3) The alkoxide product of the Grignard reaction is converted to 2-methyl-2-hexanol by treatment with aqueous NH4Cl instead of with a dilute mineral acid. The reason is that the final product is a tertiary alcohol (R3COH) and is easily dehydrated to an alkene by a strong acid. When the magnesium alkoxide is poured into aqueous NH4Cl, the alkoxide ion (a strong base) reacts with water or NH4+ to extract a proton. Water alone is not used as a hydrolyzing agent for two reasons. First, the product hydroxide ion is only a slightly weaker base than the alkoxide ion. The addition of an acid results in a more favorable equilibrium. Second, in alkaline solution, the magnesium ions are converted to a gelatinous precipitate of Mg(OH)2, which is difficult to remove from the product. In a neutral or acidic medium, the magnesium ions remain in solution. The product alcohol is extracted from the aqueous layer with diethyl ether (solvent grade). The aqueous layer, which contains the magnesium salts, is discarded. The ether solution is washed with sodium carbonate solution to ensure alkalinity prior to distillation. (Any acid remaining in the ether layer would cause dehydration of the alcohol during the distillation.) Because diethyl ether can dissolve a considerable amount of water (1.2 g H2O in 100 g of ether), the ether extract is partially dried by extraction with saturated NaCl solution before an inorganic drying agent is used. The final drying is accomplished by allowing the ether solution to stand over anhydrous MgSO4. The bulk of the ether is removed by simple distillation. Before the alcohol is distilled, the residue is transferred to a smaller distillation flask; otherwise, a considerable amount of product would be lost as vapor filling the large flask. EQUIPMENT: 400-mL beaker calcium chloride drying tube Claisen head condenser disposable pipet 25-mL or 50-mL tared distillation receiving flask 125-mL dropping funnel 50-mL, 125-mL, and two 250-mL Erlenmeyer flasks heating mantle and rheostat (or steam bath) ice bath 50-mL (or 100-mL) and 250-mL round-bottom flasks 32 250-mL or 400-mL separatory funnel simple distillation apparatus stirring rod warm water bath CHEMICALS: ammonium chloride, 25 g anhydrous acetone, 5.8 g anhydrous diethyl ether, 50 mL anhydrous magnesium sulfate or potassium carbonate, 5 g 10% aqueous sodium carbonate, 25 mL 1-bromobutane, 13.7 g diethyl ether (for extraction), about 75 mL magnesium turnings, 2.4 g saturated aqueous NaC1, 25 mL TIME REQUIRED: 3½ hours, plus 1½ hours for the distillation. The Grignard reagent must be used immediately after its formation. Therefore, enough time should be allotted to carry out Steps 1 and 2 (about 1 hour each) in a single laboratory period. IMPORTANT: If anhydrous acetone is not available, then reagent acetone must be dried with anhydrous MgSO4 (5 g for each 50 mL) for at least 24 hours prior to the Grignard reaction (see Experimental Note 4). STOPPING POINTS: after the acetone has been added to the Grignard reagent (and reaction has subsided); when the ether extracts are drying >>SAFETY NOTE I Diethyl ether (bp 34.6̊) is used as a reaction solvent and as an extraction solvent. It is very volatile and extremely flammable. There must be no flames in the laboratory. An efficient condenser must be used for both the reaction and the distillation. The distillation should be carried out slowly to minimize ether vapors escaping into the room. >> SAFETY NOTE 2 Because the formation of a Grignard reagent and a Grignard reaction are both exothermic, there is the danger of a runaway reaction. Keep an ice bath handy at all times in case the reaction flask needs rapid cooling. >>SAFETY NOTE 3 The heavy caked precipitate that sometimes forms makes thorough mixing of acetone and the Grignard reagent difficult and can allow unreacted acetone to accumulate in one spot. When this acetone eventually contacts the Grignard reagent, the reaction may become impossible to control. Therefore, swirl the reaction flask gently, but frequently and thoroughly. PROCEDURE Step 1, Preparation of n-Butylmagnesium Bromide. Heat a 250-mL round-bottom flask, a Claisen head, a condenser, and a dropping funnel in a drying oven until they are hot to the hand. Then assemble them as shown in Figure 10. Fit the reflux condenser with a drying tube containing anhydrous calcium chloride (see Experimental Note 1). To prevent atmospheric moisture from condensing inside the condenser, do not turn on the condenser water until the reaction is initiated. 33 Place 2.4 g of oven-dried magnesium turnings in the round-bottom flask. To the dropping funnel, add a well-mixed solution of 13.7 g of 1-bromobutane and 50 mL of anhydrous diethyl ether. (CAUTION: flammable! See Safety Note 1; see also Experimental Note 2.) To initiate the reaction, add 10-15 mL of the ether solution from the dropping funnel to the reaction flask. Loosen the clamp holding the round-bottom flask and gently swirl the flask to mix the contents. When the Grignard reagent begins to form, the ether solution will become cloudy and then begin to boil. Turn on the condenser water at this time. If your Grignard reagent does not start to form within 5-10 minutes, follow the procedure outlined in Experimental Note 3. Because the reaction is exothermic once initiated, do not add an excessive amount of 1-bromobutane to the magnesium at any one time (see Safety Note 2). After the reaction has been initiated, add the remaining ether solution dropwise at a rate that maintains a gentle reflux. After all the solution has been added, close the stopcock of the dropping funnel and heat the mixture at a gentle reflux for 15 minutes in a warm (50̊C) water bath. As the magnesium is consumed, the mixture will become darker colored. At the end of the reflux period, proceed immediately to Step 2. Step 2, Reaction of n-Butylmagnesium Bromide with Acetone. Chill the flask containing the Grignard reagent with an ice bath. Pour 5.8 g of anhydrous acetone (not ordinary reagent grade) in the dropping funnel, and add it a few drops at a time to the reaction mixture. After each addition, loosen the clamps to the reaction assembly and gently swirl the reaction flask. (CAUTION: See Safety Note 3.) When the addition of the acetone is completed, allow the reaction mixture to stand at room temperature for 30 minutes or longer before going on to the hydrolysis step. If the mixture will be standing for more than an hour, stopper the reaction flask with a glass stopper or a cork (not a rubber stopper) to prevent the solvent from evaporating. Step 3, Hydrolysis and Purification. Prepare 100 mL of 25% aqueous ammonium chloride. Mix 75 mL of this solution with 50 g of crushed ice in a 400-mL beaker. Transfer the remaining 25 mL of the ammonium chloride solution to a 50-mL Erlenmeyer flask, and chill it in an ice bath. Slowly pour the Grignard reaction mixture into the ice mixture in the beaker, stirring vigorously (see Experimental Note 4). Rinse the reaction vessel into the ice mixture, first with the 25 mL of chilled NH4C1 solution, then with 25 mL of solvent ether. Transfer the contents of the beaker to a 400-mL separatory funnel. (If a 250-mL separatory funnel must be used, divide the mixture into two batches and process each separately.) Add solvent ether to bring the volume of the upper ether layer to about 50 mL, shake the funnel, and allow the layers to separate. Drain the lower aqueous layer into a 250mL Erlenmeyer flask or a second separatory funnel and drain the ether layer into a separate Erlenmeyer flask. (Draining instead of pouring minimizes evaporation of the ether.) Return the aqueous layer to the separatory funnel, add 25 mL of fresh solvent ether, and shake the mixture again. Drain and discard the lower, aqueous layer. Add the first 50-mL ether extract to the second extract in the separatory funnel. Rinse the flask that contained the original extract into the separatory funnel with a few mL of ether. Wash the combined ether extracts by shaking them with 25 mL of water, then with 25 mL of 10% sodium carbonate solution. Finally, wash the ether solution with 20-25 mL of saturated sodium chloride solution. 34 Pour the ether solution into a clean, dry 250-mL Edenmeyer flask, add 5 g of anhydrous magnesium sulfate or potassium carbonate, cork the flask tightly, and allow it to stand for at least one hour (overnight is better). Carefully decant (or filter through a small plug of glass wool) the dried solution into a 250-mL roundbottom flask for distillation of the ether. Add a few boiling chips and slowly distil the bulk of the ether (bp 34.6̊C), using a steam bath or heating mantle and an efficient condenser. Stop the distillation when there is about 25 mL remaining in the distillation flask. Cool the flask and, using a disposable pipet, transfer the residue to a 50-mL round-bottom flask for distillation of the product. Wash the last traces of crude product from the 250-mL flask into the 50-mL flask with a few mL of anhydrous diethyl ether. Add fresh boiling chips and distil the product, collecting the fraction boiling at 135-143̊C in a tared receiver. A typical yield is 5.0 g (43%). (The yield may vary considerably, depending on the degree of dryness of the anhydrous ether used in Step 1.) Determine the refractive index of the product. Place the distilled product in a correctly labeled vial, and hand it in to your instructor. EXPERIMENTAL NOTES 1) The purpose of the drying tube is to prevent atmospheric moisture from entering the reaction vessel via the condenser and yet allow the reaction vessel to be open to the atmosphere so that gas pressure does not build up. There are two types of drying tubes: curved (better) and straight (less expensive). A straight drying tube must not be connected directly to the top of the condenser because the dessicant can liquefy and drain into the condenser. Connect the straight tube to the condenser by a short length of heavy-walled rubber tubing, as shown in Figure 10. In either type of drying tube, the dessicant is held in place with loose plugs of glass wool. A one-hole rubber stopper may be used as a secondary plug at the wide end of the drying tube. 2) Solvent ether contains an appreciable amount of water (up to 1-2%) and is totally unsuitable as a Grignard solvent. Anesthesia ether contains ethanol, which makes it also unsuitable. Commercial anhydrous ether is adequate only if a freshly opened can is used. Anhydrous ether must not be left open to the air because it absorbs both oxygen and moisture. (Oxygen and ethers yield peroxides, which can explode if the ether is distilled. Absorbed moisture will ruin a Grignard reagent.) Your instructor will probably provide anhydrous ether for this experiment. In many laboratories, storeroom personnel prepare anhydrous ether by passing solvent ether through a column containing molecular sieves, which are adsorbents with pores that trap molecules of a certain size (in this case, H2O molecules). Another procedure for the preparation of anhydrous ether from solvent ether and a procedure for the testing of peroxides in anhydrous ether follow. If you find it necessary to prepare your own anhydrous ether, allot an additional laboratory period. Preparation of Anhydrous Diethyl Ether. With cooling, mix a 2:1 ratio of solvent ether and conc. H2SO4 in a large round-bottom flask, and distill about two-thirds of the ether. (Do not distill all the ether.) Any water and ethanol contaminating the ether will remain with the sulfuric acid. To discard the residue, pour it onto cracked ice, allow the residual ether to evaporate in the hood, then dilute the aqueous acid with water and pour it down the hood drain with additional water. Add freshly prepared sodium wire or ribbon to the distilled ether, then allow the ether to stand at least overnight in the fume hood with the fan on. Stopper the container with a very loose-fitting cork or drying tube to allow the hydrogen gas to escape. 35 Sodium wire or ribbon is prepared by pressing sodium metal through a die, using a press. If a sodium press is not available, the ether can be dried with finely diced sodium; however, diced sodium is inferior to wire or ribbon. Another method is to add a few grams of CaCl2 to the ether and allow the mixture to stand until hydrogen has ceased to be evolved. The dried ether can then be decanted or (better) pipetted, using a rubber bulb, as needed. Commercial anhydrous ether can be further dried with sodium wire without the sulfuric acid purification step. Peroxide Test. Shake 5 mL of ether with a solution of 1 mg of sodium dichromate and one drop of dilute H2SO4 in 1 mL of water in a corked test tube. If the ether layer turns blue (from perchromate ion), peroxides are present and must be removed. Peroxide Removal. Shake the peroxide-contaminated ether with 5% aqueous ferrous sulfate (FeSO4) solution acidified with H2SO4. The iron(II) ions are oxidized with concurrent reduction of the peroxide. Aqueous sodium sulfite (Na2SO3) can be substituted for the ferrous sulfate solution. 3) The most common cause of failure of initiation of the reaction leading to the Grignard reagent is moisture (in the apparatus, in the ether, or on the magnesium turnings). In addition, in a humid atmosphere, water will collect on the sides of a cold condenser. If the initial cloudiness becomes a white precipitate, then the Mg is being converted to Mg(OH)2 by the water, and not to RMgX. If excessive moisture is present, it is best to begin anew with dry equipment and reagents. Sometimes, Grignard reagents are reluctant to form because of a magnesium oxide coating on the metal turnings. The following procedure can often overcome this difficulty. First, warm the reaction flask with a pan of warm water (about 50-60̊C). This warming will cause the ether to boil (not a sign of initiation, in this case). Remove the warm water bath and watch for the signs of initiation (spontaneous boiling of the ether). This warming may be repeated if initiation does not occur. If repeated warming does not initiate Grignard-reagent formation, add an additional 5 mL of the 1bromobutane solution from the dropping funnel and warm the flask again. As a last resort, another reagent may be added to activate the surface of the magnesium and/or indirectly complex with any water present. A number of reagents are useful: a crystal of I2, a few drops of Br2, 1.0 mL of iodomethane (methyl iodide) or dibromomethane (methylene bromide). (Only one, not all, of these should be added.) Add the reagent directly to the reaction mixture without swirling, then warm the flask in the water bath. The two inorganic halogen compounds function by reacting with the magnesium to yield an anhydrous magnesium halide, which complexes with any water present. Iodomethane and dibromomethane are reactive organohalogen compounds that react with the magnesium in slightly different ways. For example, iodomethane first forms a Grignard reagent (even when a less reactive alkyl halide does not react), which then reacts with any water present and thus removes it from solution. 36 4) The reaction mixture may contain small pieces of unreacted magnesium metal. If possible, avoid transferring these bits of metal to the ice mixture. However, a tiny piece of magnesium that cannot be removed easily from the ice mixture will do no harm. 37 Name DATE ________________ SYNTHESIS OF 2-METHYL-2-HEXANOL: A GRIGNARD REACTION: WORKSHEET Mass of Product Ref. Index CRC Ref. Index Theoretical Yield (grams) Actual Yield (Percent) Attach the I.R. Spectrum of the Product Locate the position of the following peaks in your spectrum; -OH peak -CH stretch -C-OH stretch PROBLEMS 1) Write equations for the three standard steps in a Grignard synthesis in which the principal reactants are cyclohexanone and ethylmagnesium bromide. 2) A student oven-dries the glassware needed for a Grignard reaction, then stores them in a locker until the next laboratory period. Will the glassware still be dry when the Grignard reaction is begun? Explain. 3) Suggest a reason for using magnesium turnings instead of magnesium powder or chunks in a Grignard reaction. 38 4) In which of the following steps in a Grignard synthesis is anhydrous ether (instead of solvent ether) necessary? Explain. (a) Preparation of the Grignard reagent. (b) Addition of an ether solution of a ketone (instead of pure ketone, as in this experiment). (c) Extracting the product from the hydrolysis mixture. (d) Washing the dried product into a distillation flask. 5) Are diethyl ether vapors lighter or heavier than air? What are the safety implications of your answer? 6) As an alternative to drying tubes to protect a Grignard reaction, some chemists carry out these reactions under a dry nitrogen atmosphere. Which of the following techniques could also be used to keep a Grignard reagent dry? Explain. (a) a dry argon atmosphere (b) a dry carbon dioxide atmosphere (c) a gentle stream of dried air passed over the surface of the mixture. 39 EXPERIMENT 8 Biodiesel Synthesis This experiment demonstrates the use of vegetable oil as an alternative, renewable fuel. The reaction incorporates NaOH as a catalyst in order to achieve high yield and minimize waste. In addition, the glycerol by-product can be reused in order to make glycerine soap. INTRODUCTION The United States is the largest single consumer of fossil fuels in the world. Each year, the U.S. consumes 125 billion gallons of gasoline and 60 billion gallons of diesel fuel. With current energy consumption, the desire to find alternative fuels for our energy needs is increasing. One such alternative fuel is vegetable oil. Vegetable oil offers the benefits of a greener synthetic route for obtaining diesel fuel. This fuel source is commonly known as biodiesel, and can be synthesized on an individual level or on an industrial scale. The methods behind biodiesel synthesis have been known for quite a while. In recent years, however, there has been significant interest in the production of biodiesel from the waste oils of the food industry. Every year, fast food restaurants in the U.S. produce over 3 billion gallons of used cooking oil. Since many gallons of this used oil inevitably end up in landfills and sewers, the production of biodiesel from waste oil has the potential to significantly reduce environmental impact. In this experiment you will synthesize diesel fuel from a triester of glycerol (a triacylglycerol or triglyceride). This reaction is known as a transesterification reaction. Transesterification is the process of transforming one type of ester into another type of ester. This reaction incorporates the use of the strong base sodium methoxide in a base- catalysed nucleophilic addition/elimination reaction at the carbonyl carbon of the triglyceride. This experiment is not entirely “Green.” The methanol used in this experiment is derived from petroleum sources. Ethanol, derived from vegetable sources like corn, could have been used but the product is less volatile and more difficult to make than the methyl ester. The overall mechanism is catalyzed by the presence of NaOH. In the first step of the reaction, NaOH reacts with methanol in an acid-base reaction. The product of this reaction is the very strong base sodium methoxide and water. In the second step, the sodium methoxide acts as a nucleophile and attacks the three carbonyl carbons of the vegetable oil. This produces a tetrahedral intermediate that is highly unstable. The overall result is the "cracking" of the triglyceride. The elimination of the glycerol backbone leads to the formation of the three methyl esters (the biodiesel) and glycerol. The NaOH is reproduced as a product in the reaction. If the biodiesel is removed from the mixture, glycerol and unreacted NaOH and methanol remain. The glycerol can be converted to soap through a saponification reaction if excess NaOH is used. Care must be exercised when using excess NaOH, because using too much will produce a jelly like mix of glycerol and soap. 40 EXPERIMENTAL PROCEDURE Note: The following procedure is for synthesizing a biodiesel mini-batch from 100% pure unused vegetable oil. This method can easily be modified for using recycled, used vegetable oil or fats like bacon grease. 1. Add 0.35 g of finely ground anhydrous NaOH into 20 mL of pure (99% or higher purity) methanol in a 250 mL Erlenmeyer flask containing a magnetic stir bar. Put the flask on a magnetic stir plate, and stir vigorously until all of the NaOH is dissolved. This flask now contains sodium methoxide. Note: Sodium methoxide is an extremely strong base and should be handled with care. 2. Warm up 100 mL of vegetable oil or grease to about 40°C in a 250 mL beaker. Warming the oil up is not necessary, but increases the reaction rate. 3. When all of the NaOH is dissolved, pour the 100 mL of oil into the methoxide solution while continually stirring. At first the mixture will become cloudy, but should soon separate into two layers. Stir for 15-30 minutes on high. (Stop here if experiment is being done over 2 weeks.) 4. Transfer the contents of the flask into a 250 mL separatory funnel. The mixture will separate into two different layers. The glycerol will fall to the bottom, and the methyl ester (biodiesel) will float to the top. Since about 75% of the separation occurs within the first hour, you will be able to see immediate progress. Allow the experiment to sit for about an hour. 5. Open the stopcock of the separatory funnel and allow the glycerol to drain into a small beaker. Make sure not to get any biodiesel in the glycerol or glycerol in the biodiesel. 6. Use the IR spectrometer to identify your products. Print out the spectras and compare with known spectra. The biodiesel may be hard to compare, since most oils are comprised of different length carbon chains. Comparing to known spectra can easily identify the glycerol. The presence of glycerol indicates a successful reaction. EXPERIMENT REPORT For this experiment I would like you to create a summary report. The report should be a typed one or two page narrative and should include: A brief summary of your experiment and results Analyze the quality and error of your experiment. Evaluate this experiment in terms of its greenness. What recommendations do you have to improve the green character of this reaction? Attach a copy of your IR (another copy should be taped in your notebook). 41 In addition, you should answer the following questions and make them a part of your report. 1. What is the reaction that is occurring that produces biodiesel? 2. Most oils and fats contain palmitic and stearic acid as building blocks. Give the structure for both these compounds. 3. Describe the role of sodium hydroxide in this reaction. 4. How “Green” is this experiment? Please answer the following, a) Where did you get your oil/fat you used in this experiment and what would have happened to it had you not converted it to biodiesel? b) What was the source of your methanol? Is methanol made from natural sources or is it a product of the oil industry? Is methanol “Green”? c) What was in the waste product and what did you with it? 42 Exam I NMR IR MS Diels-Alder 43 Chem 241 Exam #1 Name___________________ February 28, 2001 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(20) Credit 2(20) 3(15) 4(10) 5(15) 6(20) TOTAL 1) Please draw the product of a 6 + 2 reaction. Draw the MO diagram indicating the orbital symmetry, the HOMO and LUMO, and whether the reaction occurs thermally or photochemically. 2) The cyclopropenyl cation is a completely conjugated ring system. Please draw the cyclopropenyl cation and its MO diagram below 44 3a) Please draw the product of each of the following Diels-Alder reactions. O O + + C O C O Cl O + 3b) One of the two dienes below can be used in a Diels-Alder reaction and the other one cannot. Which one CANNOT be used and why? A. B. 4) Please draw the MO diagram for each of the following compounds. Circle the aromatic compounds (if any). H 5) Please explain the endo rule and give an example 45 N Chem 241 Exam #1 Name___________________ February 28, 2001 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(20) Credit 2(20) 3(15) 4(10) 5(15) 6(20) TOTAL 1) Please draw the product of a 6 + 2 reaction. Draw the MO diagram indicating the orbital symmetry, the HOMO and LUMO, and whether the reaction occurs thermally or photochemically. +-+-+- The HOMO of the 2 matches the LUMO of the 6 only if an electron is photochemically moved. + - ++ - + - + - Thefore the reaction occurs photochemically +--++- + Zero of Energy - + + HOMO +- LUMO ++--++ - ++ +++--- ++++++ 2) The cyclopropenyl cation is a completely conjugated ring system. Please draw the cyclopropenyl cation and its MO diagram below H H H- 46 3a) Please draw the product of each of the following Diels-Alder reactions. O O O O + Cl + C C Cl O O O O O + O 3b) One of the two dienes below can be used in a Diels-Alder reaction and the other one cannot. Which one CANNOT be used and why? Rotate A. These groups hinder one another so the molecule does not want to take this shape. Rotate B. These groups do not hinder one another so the molecule can take this shape and do a Diels Alder. 4) Please draw the MO diagram for each of the following compounds. Circle the aromatic compounds (if any). H N 5) Please explain the endo rule and give an example Cl The endo rule says that the electron withdrawing group on the dienophile must overlap with the double bond from the diene (toward the interior of the molecule) + 47 C C Cl Chem 241 Practice Exam #1 – NMR/IR/MS/MO Theory Name___________________ February 28, 2001 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(20) Credit 2(20) 3(15) 4(10) 5(15) 6(20) TOTAL 1a) Using drawings, please show how a triplet is formed in NMR spectroscopy. 1b) What are the relative peak heights in a heptet? Show them as a ratio, ie. 1:2:1 etc. 1c) Please draw the complex NMR splitting diagram for the following molecule. JAB = 11, JBC = 9. Along with your diagram indicate where the signal would be found in the spectrum. H Cl A C H H B C H H C C H H 48 1d) Look at the complex splitting pattern shown below 10 2 8 2 2 6 2 2 8 2 10 What is the value of JAB and JBC? JAB = JBC = JAB is a singlet, doublet, triplet, quartet (circle one) JBC is a singlet, doublet, triplet, quartet (circle one) 2a) Please explain why alkenes are found at 5-6 in an NMR but alkynes are found around 2.5. 2b) In NMR an alcohol and an acid OH differ in position by 8ppm. Please give two reasons why the acid OH is so much farther downfield. 3) Please draw the product of a 4 + 4 reaction. Draw the MO diagram indicating the orbital symmetry, the HOMO and LUMO, and if the reaction occurs thermally or photochemically. 4a) In Mass Spectrometry what are the approximate relative peak heights (in percent) for the following halogens, 35 Cl = 79 Br = 37 Cl = 81 Br = 49 4b) In IR the C-Cl bond is found at 754 cm-1 and the C-Br stretch is found at 548 cm-1. Which of these has the stronger force constant and by how much? C = 12g/mol, Cl = 35.45g/mol, Br = 79.9 g/mole 5) Please explain the endo rule and give an example. 6) Please draw the product of the following Diels-Alder reactions. C C O O C C C + CH3 + Cl C C 50 Chem 241 Practice Exam #1 – NMR/IR/MS/MO Theory Name___________________ February 28, 2001 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(20) Credit 2(20) 3(15) 4(10) 5(15) 6(20) TOTAL 1a) Using drawings, please show how a triplet is formed in NMR spectroscopy. 1:2:1 1b) What are the relative peak heights in a heptet? Show them as a ratio, ie. 1:2:1 etc. Singlet 1 Doublet 1 1 Triplet 1 2 1 Quartet 1 3 3 1 Pentet 1 4 6 4 1 Hextet 1 5 10 10 5 1 Heptet 1 6 15 20 15 6 1 1c) Please draw the complex NMR splitting diagram for the following molecule. JAB = 11, JBC = 9. Along with your diagram indicate where the signal would be found in the spectrum. ~ 2.0 H Cl A C H H B C H H C C H 11 H 9 4 5 51 4 11 4 1 4 4 5 4 9 1d) Look at the complex splitting pattern shown below 10 2 8 2 2 6 2 2 8 2 10 What is the value of JAB and JBC? JAB = 12 JBC = 10 JAB is a singlet, doublet, triplet, quartet (circle one) JBC is a singlet, doublet, triplet, quartet (circle one) 2a) Please explain why alkenes are found at 5-6 in an NMR but alkynes are found around 2.5. H H C H C H C C H H Since the ring current in an alkene pulls electrons away from the hydrogens they are pulled downfield as compared to an alkyne whose circulating electrons help shield its hydrogens. 2b) In NMR an alcohol and an acid OH differ in position by 8ppm. Please give two reasons why the acid OH is so much farther downfield. a) The extra oxygen on an acid pulls more electron density out of the OH bond forcing it further downfield. b) An acid group has a C=O which sets up ring currents that pull electrons out of the atoms to which they are attached and this pulls the OH group farther downfield. 52 3) Please draw the product of a 4 + 4 reaction. Draw the MO diagram indicating the orbital symmetry, the HOMO and LUMO, and if the reaction occurs thermally or photochemically. +-++ LUMO + - +-+Symmetry match + +--+ - HOMO --+ Photochemically induced promotion Zero of Energy - + ++ -- ++ -- + ++++ ++++ If an electron is photochemically moved to the next highest orbit, the symmetry of the new HOMO matches the LUMO of its reacting partner. 4a) In Mass Spectrometry what are the approximate relative peak heights (in percent) for the following halogens, 35 37 79 81 Cl = 75% Br = 50% Cl = 25% Br = 50% 4b) In IR the C-Cl bond is found at 754 cm-1 and the C-Br stretch is found at 548 cm-1. Which of these has the stronger force constant and by how much? C = 12g/mol, Cl = 35.45g/mol, Br = 79.9 g/mole 12 79.9 k Cl v1 k k 754 12 79.9 1 2 Cl 1.626 v2 k2 1 548 k Br 12 35.45 k Br 12 35.45 Therefore, the Cl force constant is 1.626 times larger than the Br. 5) Please explain the endo rule and give an example. The endo rule says that we should place the electron-withdrawing group on the dienophile in such a way that it is stabilized by the p-orbital overlap on the diene. The essentially places the electron withdrawing to the “inside of the diene. Cl Cl H3C H3C This way is endo. The Cl is not stabilized This way is endo. The Cl is stabilized. 6) Please draw the product of the following Diels-Alder reactions. C C C C O O O C + O Cl C C CH3 + Cl C C CH3 53 Chem 241 Practice Exam #1 Name___________________ February 28, 2000 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(12 ) Credit 2(24) 3(24) 4(10) 5(15) 6(15) TOTAL 1a) What is the possible explanation for the differences in the postion of the aromatic protons in the following compounds? Benzene = 7.37 Toluene = 7.17 p Xylene = 7.05 1b) Please draw the complex splitting diagram for carbon B in the following compound. Where will the signal be centered? Jab = 11 Hz and Jbc = 9 Hz Br C C C C A B C Cl 54 2) Approximately where would you expect to find a C=S bond in an I.R. spectrum? Show your work and state your assumptions. C= 12, S=32, O=16 3) Please draw the MO diagram for a 6+2 reaction showing the symmetry of all energy levels, where the electrons go, and label the HOMO and LUMO for each. Will the reaction occur photochemically or thermally? 4) Choose any two of the NMR/IR/MS spectras on the next page and determine the structure of the compound. Draw the compounds here. 55 5a) Please explain the difference between the position of a double bond versus a triple bond in NMR. 5b) I am sure that you know that the hydrogens on C=C are not as far down field as those on C=O, which are not as far downfield as those found on an acid group, COOH. Please explain this observation. Give at least two separate and unrelated reasons why this is so. 6) Please draw the product of the following Diels-Alder reactions; O O O O 2x 56 Chem 241 Practice Exam #1 Name___________________ February 28, 2000 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(12 ) Credit 2(24) 3(24) 4(10) 5(15) 6(15) TOTAL 1a) What is the possible explanation for the differences in the postion of the aromatic protons in the following compounds? Benzene = 7.37 Toluene has one methyl group, Xylene has two methyl groups and Benzene has none. Methyl groups push electrons into the ring and these electrons help shield the ring from the effects of the externally applied magnetic field and this pushes the signal upfield --> Toluene = 7.17 p Xylene = 7.05 1b) Please draw the complex splitting diagram for carbon B in the following compound. Where will the signal be centered? Jab = 11 Hz and Jbc = 9 Hz Br C ~2.0 C C C A B C Cl 11 9 7 2 57 2 9 2) Approximately where would you expect to find a C=S bond in an I.R. spectrum? Show your work and state your assumptions. C= 12, S=32, O=16 Since both of them have double bonds, assume k1 = k2 which means that they will cancel, so the only difference is the mass effect (). Since C=O is at 1710 cm-1 use it as 1. v1 v2 12 32 1710 12 32 v2 1516cm 1 v2 12 16 12 16 k1 2 k2 1 3) Please draw the MO diagram for a 6+2 reaction showing the symmetry of all energy levels, where the electrons go, and label the HOMO and LUMO for each. Will the reaction occur photochemically or thermally? +-+-++-++-+ HOMO ++--++++--++ LUMO Photon + ++ +++--++++++ The reaction occurs photochemically 4) Choose any two of the NMR/IR/MS spectras on the next page and determine the structure of the compound. Draw the compounds here. 58 5a) Please explain the difference between the position of a double bond versus a triple bond in NMR. H H C C H H C C H H Since the ring current in an alkene pulls electrons away from the hydrogens they are pulled downfield as compared to an alkyne whose circulating electrons help shield its hydrogens. 5b) I am sure that you know that the hydrogens on C=C are not as far down field as those on C=O, which are not as far downfield as those found on an acid group, COOH. Please explain this observation. Give at least two separate and unrelated reasons why this is so. a) The ring current in the double bond push all of them down field. b) The electron withdrawing effect of the C=O pushes the hydrogens on this carbon farther down field. c) Since the hydrogen in an acid group is directly attached to the electron withdrawing oxygen and there is a nearby C=O that causes a shift downfield, the acids hydrogen is pushed farthest downfield of the three. 6) Please draw the product of the following Diels-Alder reactions; O O O O O O O O 2x 59 Chem 241 Practice Exam #1 Name___________________ February 25, 1994 Closed Book Exam - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(16 ) Credit 2(40) 3(16) 4(12) 5(16) TOTAL 1) One of the proton environments in an organic compound produces the following complex splitting pattern. What is the value for Jab and Jac for this compound? Show how this pattern arises and label the splitting diagram. 2) Please draw the MO diagram each of the following compounds, O 60 3) Choose any one of the following NMR / IR’s and draw the compound. 4a) Cyclopentadiene is not aromatic because it is not completely conjugated and it is not flat. There are three forms of the molecule that are completely conjugated and one of them is aromatic. Please draw all three forms and indicate which one is aromatic. 4b) Please draw the MO diagram for each of the molecules you drew above. 61 Chem 241 Practice Exam #1 Name___________________ February 25, 1994 Closed Book Exam - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(16 ) Credit 2(40) 3(16) 4(12) 5(16) TOTAL 1) One of the proton environments in an organic compound produces the following complex splitting pattern. What is the value for Jab and Jac for this compound? Show how this pattern arises and label the splitting diagram. Jab = 10 Jac = 14 2) Please draw the MO diagram each of the following compounds, O 62 3) Choose any one of the following NMR / IR’s and draw the compound. 4a) Cyclopentadiene is not aromatic because it is not completely conjugated and it is not flat. There are three forms of the molecule that are completely conjugated and one of them is aromatic. Please draw all three forms and indicate which one is aromatic. Remove H+ Remove H Remove H- Aromatic 4b) Please draw the MO diagram for each of the molecules you drew above. See above 63 Exam II Aromatics Electrophilic Substitution MO Theory Hückel Reaction 64 Activators and Deactivators on Benzene O/P Activators by Induction O/P Activators by Resonance CH3 OH C2H5 NH2 C(CH3)3 NHR CH(CH3)2 NR2 OCH3 O O/P Deactivators w/ Resonance O C CH3 Meta Deactivators by Induction F NO2 Cl HSO3 Br COOH I CN CHO NH3+ Note: There is no such this as a meta activator 65 Resonance forms of O/P Activators by Resonance +O H E A OH OH OH OH A E+ Ortho E + E E B B + OH + OH OH E+ OH + + Meta E + OH E OH E OH OH A E+ + Para B B + + E E E A +O H E 66 Resonance forms of O/P Deactivators - Halogens + Cl E A Cl Cl Cl Cl A E+ Ortho E + E E B B + Cl + Cl Cl E+ Cl + + Meta E + Cl E Cl E Cl Cl A E+ + Para B B + + E E E A + Cl E 67 Resonance forms of O/P Activators by Induction – Alkyl Groups CH3 CH3 E+ CH3 E + CH3 E E Ortho + CH3 CH3 + CH3 E+ CH3 + + Meta E + CH3 E CH3 E CH3 E+ CH3 + Para + + E E E Resonance forms of Meta Deactivators by Induction NO2 NO2 E+ NO2 E + NO2 E E Ortho + NO2 NO2 + NO2 E+ NO2 + + Meta E + NO2 NO2 E E NO2 E+ NO2 + Para + + E E 68 E Reactions of Benzene – Electrophilic Substitution Cl 2,FeCl 3 X = Cl X Br2, FeBr3 Cl 2O, CF 3COOH I2, CuCl 2 X = Br X = Cl X=I NO2 HNO 3, H2SO4 Nitration SO3H Fuming H2SO4 Sulfonation OH H2O2, HSO3F Hydroxylation C2H5 C2H5Cl, AlCl 3 + HCl Friedel Crafts Alkylation O C CH3 CH 3COCl , AlCl 3 + HCl 69 Friedel Crafts Acylation Benzene Side Group Reactions O R R C H2C Zn(Hg) Dilute HCl NH2 NO2 KMnO4, H2O Heat NO2 NH2 Will not reduce other reducible side groups like aldehydes and acids. 1. SnCl2, HCl 2. NaOH, H2O CH3 CH2Br NBS HSO3 OH Conc. NaOH Heat COOH CH2OH 1. LiAlH4, THF 2. H+ Cl OH 1. NaOH, Heat 2. H2O, H+ 70 Chem 241 Exam #2 Name___________________ April 3, 2000 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(12) Credit 2(12) 3(12) 4(10) 5(10) 6(16) 7(28) TOTAL 1) Please name each of the following compounds. NH2 Cl CH3 HO COOH H3C O O O O Cl 71 2) For each of the following compounds label them as either an activator or deactivator, and as an ortho, para, or meta director. HC O NH2 Cl Act/Deact ________ ________ ________ Director ________ ________ ________ 3a) Please draw all of the resonance structures for ortho attack of chlorobenzene with an electrophile (E+). 3b Please draw all of the resonance structures for ortho attack of phenol with the chloronium cation. Circle the most stable strcture. 4) Please draw the MO diagram each of the following compounds, O 72 5) Please draw the complete mechanism for the Friedel-Craft alkylation of benzene using bromobutane as the alkylating agent. 6a) Please synthesize both of the compounds given below. You may start with benzene, an alcohol, alkyl halide, or alkane. Neither of these compounds require more than five steps to synthesize. C C C NH2 6a) Please name three criteria that determines whether a molecule is aromatic. 6b) Cyclopropene is not aromatic but it can be made aromatic. Please draw the aromatic form of cyclopropene. 6c) Can 1,3-cyclobutadiene be made aromatic? Why or why not? Use examples. 73 7) Please complete the following reactions. If there is no reaction, write “No Reaction.” CH3 KMnO 4, H2SO4, Heat NHCH2CH3 AlCl3, C-C-Cl 1. CO, HCl, AlCl3 2. HNO3, H2SO4 O C C C H2SO4, HO-C- C-OH OH C C C 1. PCC 2. C-C-MgCl 3. H2O O C C C N2H4, NaOH, H 2O C C C C O C KOH, H 2O 74 Chem 241 Exam #2 Name___________________ April 3, 2000 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(12) Credit 2(12) 3(12) 4(10) 5(10) 6(16) 7(28) TOTAL 1) Please name each of the following compounds. NH2 Cl CH3 HO COOH H3C Aniline m-methyltoluene (m-xylene) 2-chloro-3-hydroxybenzoic acid O O O O Cl Phenanthrene 1,3-cyclobutadione 75 3-chloro-6-oxo-2-hexanone 4-chloro-5-oxohexanal 2) For each of the following compounds label them as either an activator or deactivator, and as an ortho, para, or meta director. HC O NH2 Cl Act/Deact _Deact__ __Act___ _Deact__ Director ___m___ __o/p___ __o/p___ 3a) Please draw all of the resonance structures for ortho attack of chlorobenzene with an electrophile + Cl (E+). E A Cl Cl Cl Cl A E+ E + E E B B + + 3b Please draw all of the resonance structures for ortho attack of phenol with the chloronium cation. Circle the most stable strcture. +O H Cl Most Stable A OH OH OH OH A + Cl Cl Cl B B + + 4) Please draw the MO diagram each of the following compounds, O 76 5) Please draw the complete mechanism for the Friedel-Craft alkylation of benzene using bromobutane as the alkylating agent. Br C C C C Br AlBr3 C C C C Al Br Br Br H C C C CH3 Hydride Shift C C H C C C C H C Br CH2 + Al Br H C C C Br Br C + H+ 6a) Please synthesize both of the compounds given below. You may start with benzene, an alcohol, alkyl halide, or alkane. Neither of these compounds require more than five steps to synthesize. C C C C C C 1. Propanoyl Chloride, AlCl3 2. H2SO4, HNO3 3. Zn(Hg), HCl NH2 NH2 O O 1. Br C C C Br 2. Zn(Hg), HCl AlBr3 6a) Please name three criteria that determines whether a molecule is aromatic. i) ii) iii) The molecule must be completely conjugated. The molecule must be flat The molecule must have 4n+2 electrons in the ring. 6b) Cyclopropene is not aromatic but it can be made aromatic. Please draw the aromatic form of cyclopropene. H H H + H- Remove the H with its electrons Produces an empty p orbital cyclopropenyl cation 6c) Can 1,3-cyclobutadiene be made aromatic? Why or why not? Use examples. No. Regardless of the number of electrons in the ring, some of the electrons will be on the zero of energy. Zero of Energy 77 7) Please complete the following reactions. If there is no reaction, write “No Reaction.” CH3 COOH KMnO 4, H2SO4, Heat NHCH2CH3 AlCl3, C-C-Cl No Reaction H C O 1. CO, HCl, AlCl3 2. HNO3, H2SO4 NO2 O C C C H2SO4, HO-C-C-OH OH C C C 1. PCC 2. C-C-MgCl 3. H2O O C C C N2H4, NaOH, H 2O C C C C O C O O OH C C C C C C C C KOH, H2O C C OH C C C O and C C C OH C C 78 Chem 241 Exam #2 Name___________________ March 29, 2006 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(12) Credit 2(12) 3(12) 4(10) 5(16) 6(14) 7(24) TOTAL 1) Please name each of the following compounds. NH2 O O2N C Cl OH Cl Cl N N 79 2) For each of the following compounds label them as either an activator or deactivator, and as an ortho, para, or meta director. H OCH 3 S C Act/Deact ________ ________ ________ Director ________ ________ ________ 3) Please draw all of the resonance structures for para attack of chlorobenzene with the nitronium cation (NO2+). Circle the most stable structure. 4) Please draw the complete mechanism for hydroxylation of toluene. 80 5a) Please name three criteria that determines whether a molecule is aromatic. 1) 2) 3) 5b) Pyrrole is an aromatic compound. Please draw the compound and explain why it is aromatic. 5c) Please draw the aromatic form of 1,3,5 cycloheptatriene. 5d) The following compound is not currently aromatic. Can it be made aromatic? If so, draw the aromatic form of the molecule. If not, explain why not. N 81 6) Please synthesize two of the three following compounds. You can start with benzene, alkyl halides or alcohols. OH OH m catechol Naphthalene O O O C CH3 C HO Aspirin 82 7) Please complete the following reactions. If there is no reaction write “No Reaction.” H C O AlCl3 CH3Cl HSO3 1. FeCl3, Cl2 2. NH3, NaNH2 1, CO, HCl, AlCl3 2. H2SO4, HNO3 3. LiAlH4 NO2 Fuming H2SO4 CH3 C-C-C-Cl AlCl3 C C C KMnO 4, H+, H2O, Cl2, 1000 psi, 300oC NH2 FeCl3 Cl 2 83 Chem 241 Exam #2 Name___________________ March 29, 2006 CLOSED BOOK EXAM - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(12) Credit 2(12) 3(12) 4(10) 5(16) 6(14) 7(24) TOTAL 1) Please name each of the following compounds. NH2 O O2N Aniline C m-nitrobenzoic acid OH Cl Cl 1,2,3-trichlorobenzene N N Anthracene Cl pyrrole pyridine 84 2) For each of the following compounds label them as either an activator or deactivator, and as an ortho, para, or meta director. H OCH3 S C Act/Deact __Act___ __Act___ __Deact_ Director __o/p__ __m____ ___o/p__ 3) Please draw all of the resonance structures for para attack of chlorobenzene with the nitronium cation (NO2+). Circle the most stable structure. Cl Cl Cl Cl A + B B + + NO2 NO2 NO2 A + Cl Most Stable NO2 4) Please draw the complete mechanism for hydroxylation of toluene. H O O H HSO3F H H+ O + O HO+ H H CH3 CH3 CH3 HO+ + H+ + HO H OH 85 + H2O 5a) Please name three criteria that determines whether a molecule is aromatic. 1) Completely conjugated ring 2) 4n + 2 electrons 3) No non-bonding orbitals 5b) Pyrrole is an aromatic compound. Please draw the compound and explain why it is aromatic. Normally, the nitrogen would be sp3 hybridized, but in pyrrole, it is sp2 hybridized so that the electrons can be in a p orbital and completely conjugate the ring giving it 6 electrons as required by Huckel’s Rule H N N N H H Normal sp3 hybridized nitrogen pyrrole Rehybrized nitrogen to sp2 5c) Please draw the aromatic form of 1,3,5 cycloheptatriene. Leaves as H- H H 1,3,5-cycloheptatriene showing the electrons in one of the bonds to hydrogen Empty p orbital H + H Remove the hydrogen with the electrons so that you don't add any more electrons to the ring. Six total electrons in the ring. Aromatic cation 5d) The following compound is not currently aromatic. Can it be made aromatic? If so, draw the aromatic form of the molecule. If not, explain why not. N This molecule cannot be made aromatic because, no matter what you do, its MO diagram will have orbitals on the zero of energy (non-bonding orbitals). 86 6) Please synthesize two of the three following compounds. You can start with benzene, alkyl halides or alcohols. OH OH m catechol Naphthalene O O O C CH3 C HO Aspirin HSO3 Fuming H2SO4 HSO3 OH conc. NaO H Heat H2O2, HSO3F Heat OH O OH OH O Cl 1. KMnO4, H+ H2O, heat 2. SOCl2 1, LiAlH4 2. SOCl2 x 2 3. tButO-, tButOH AlCl3 Cl OH O O O O 1. CH3Cl, AlCl3 HO 2. H2O2, HSO3F, Heat 3. KMnO4, H+, H2O, Heat OH C O C Acetic Anhydride H2SO4, H2O, Heat HO 87 O C CH3 7) Please complete the following reactions. If there is no reaction write “No Reaction.” H C O AlCl3 No Reaction - the ring is deactivated CH3Cl HSO3 NH2 1. FeCl3, Cl2 2. NH3, NaNH2 NH2 CH3 1, CO, HCl, AlCl3 2. H2SO4, HNO3 3. LiAlH4 NH2 NO2 NO2 Fuming H2SO4 CH3 HO3S CH3 C C C C-C-C-Cl AlCl3 C C C COOH KMnO 4, H+, H2O, Cl2, 1000 psi, 300oC Cl H H Cl H Cl H Cl H Cl NH2 NH2 Cl H FeCl3 Cl 2 Cl 88 Lindane - a pesticide Exam III Carbonyl Chemistry Acids, Esters, Aldehydes, Ketones, Amides, Nitriles The chemistry of carbonyls is broken down into four main groups; 1. Substitution - Aldehyde and Ketone Chemistry 2. Addition – Acid and Acid Derivative Chemistry 3. Conjugate Addition - Acid Derivatives, Ketones, & Aldehyde 4. Alpha Substution – Acid Derivatives Each of these four types of reactions are shown on the next page. We will study them one at a time. In each case we will learn how to make the compounds in which we are interested and then apply one of these four reactions to them. 89 Carbonyl Chemistry Four Main Reaction Types 1. Addition – Aldehydes and Ketones O- O H3C C CH3 OH H2O Nuc- H3C C CH3 H3C C Nuc CH3 + OH- Nuc 2. Substitution – Acid & Derivatives O- O H3C C Cl Nuc- H3C O C H3C Cl + Cl- C Nuc Nuc 3. Conjugate Addition – All Carbonyls O C C C OCH3 Nuc- C C O OH C CH3 H2O Nuc C C C CH3 keto-enol C C Nuc Nuc 4. Alpha Substitution – All Carbonyls O O Base- H3C C CH3 H3C O CH2- C CH 3Br H3C - (Nuc ) 90 C C CH3 + Br- C CH3 How to make Aldehydes and Ketones C C OH PCC CH2Cl2 C C C C PCC CH2Cl2 C CH C KMnO 4, H+, H2O C C C C O OH C C O OH C O O OH C C C K2Cr2O7, H2SO4, Heat C C C O O3, (CH 3)2S C C O C C O O C C + C C C O C C C C OH DIBAH, -70C C BH3, H2O2, NaOH, H 2O C H2SO4, HgSO4, H2O C O C C C C2H5MgBr C C O O C C Cl C C C2H5 C C O O C C Cl Li(t-ButO)3AlH HC O CO, HCl, AlCl3 Gatterman-Koch Rxn R RCOCl , AlCl 3 C O Friedel-Craft Acylation 91 1. Nucleophilic Addition Reactions on Aldehydes and Ketones General Mechanism - O C O - C Nuc C C C OH H2O C C C Nuc Nuc Reactions O C C OH C HCN C C C Cyanohydrin CN OCH3 OH CH 3OH H+, H2O C C C CH 2OH H+, H2O Acetal Hemiacetal OH C C C Gemdiol OH OH 1. C2H5MgBr 2. H2O, H+ C C C C2H5 OH LiAlH4, H2O C C C OH NaBH4, H2O C C C OH H2/Pd/BaSO 4/S Rosenmund Catalyst C C C C OCH3 OCH3 OH-, H2O C C 92 C Aldehyde / Ketone Amine Reactions O C C NH C NH 3 NH2 N N2H4 C C C Imine C C C Hydrozone Hydrazine OH N NH 2OH C C C Oxime Hydroxylamine R N NH 2R C C C Imine "Schiff Base" Amine NHPh N PhNHNH 2 C C C Phenylhydrozone Phenylhydrazine NHCONH2 N NH 2CON 2H4 C C C Semicarbazone Semicarbizide Aldehyde/Ketone Name Reactions O C C C Zn(Hg), HCl C C C Clemmenson Reduction C C C Wolff-Kishner Reduction O C C H C N2H4, KOH HO O O H C C KOH, H 2O + 93 CH OH Cannizaro Reaction How to make Acids C C C KMnO 4, Heat OH C C O K2Cr2O7, H2SO4 C C C KMnO 4, Heat C C N O C OH O C C 1, I2, NaOH 2. H+ C H3C C H+, H2O, Heat 1. Mg, Ether 2. CO2 3. H+ Br 2. Nucleophilic Substitution Reactions on Acids General Mechanism O- O H3C C X Nuc- H3C C O H3C X Nuc Nuc Formation of Acid Halides SOCl 2 O O POCl 3 CH3 C CH3 C OH PCl3 Cl or Br PBr3 Formation of Anhydrides O CH3 C O C CH3 HO O O Cl O O CH3 C O C CH3 HO CH3 C OH + X- C H2SO4 94 C H3C Formation of Esters O CH3 C C2H5OH O Cl CH3 C O CH2 CH3 N CH2 CH3 O C2H5OH, H2SO4 CH3 C OH Formation of Amides O CH3 C C2H5NH 2 O Cl CH3 C O H C2H5NH 2 CH3 C OH Formation of Nitriles O CH3 C NH2 POCl 3 or P2O5 CH3 H3C Br NH2 C N HCN 1. NaNO2, HCl 2. CuCN CN 95 Sandmeyer Reaction 3. Conjugate Addition Using Esters, Diones, and Enones General Michael Reaction Enone C C Nucleophile O C Nuc- R OC C C O OH R H2O Nuc C C C keto-enol R C Nucleophiles R = diester dione Keto-ester O -CH3 Ketone -O-R Ester -H Aldehyde -NH2 Amide Lewis Acceptors (acids) C R Nuc Nuc Enones C H3C O O C H3C CH 2 H3C O H3C C CH 2 H3C diester O CH 2 H3C O Lewis Donors (bases) O C O O dione ketoester Therefore, if R = CH3 and Nuc- = Keto-ester, the product would be; O O H3C O H3C O C CH2 + C C C O O C C CH3 C C C CH3 H3C H3C O O If the ester is hydrolyzed then you get a spontaneous decarboxylation of a β keto acid; O O H3C O CH3OH + HO O C C H3C C C C CH3 C C H+, H2O H+ + O O C H3C C C C O CH3 C H3C O O The mechanism of the decarboxylation is in your notes. 96 O O C C C CH3 4. Alpha Substitutions - Claisen Reactions Classic Claisen Reaction - between two of the same esters O H3C O C O NaNH 2 CH3 H3C O C O- O CH2- H3C O C H3C CH3 H3C O O C CH3 CH2 C O O O O CO2 + H+ + C CH3 CH3O + C spontaneous decarboxylation CH3 - CH3OH + HO C CH3 CH2 H+, H2O H3C Heat O C CH3 CH2 C O O keto acid Acetone Crossed Claisen – between two different esters O H3C O C O NaNH 2 CH3 H3C O C O- O CH2- H3C O C CH2 CH3 H3C H3C O O C CH2 CH3 CH2 C O O O O CO2 + H+ + C CH2 CH3 CH3O + C C spontaneous decarboxylation CH3 - CH3OH + HO C CH2 CH3 H+, H2O Heat CH2 H3C O C CH2 CH3 CH2 O O Methyl ethyl ketone ( 2 propanone) keto acid Dieckmann Reaction – A Claisen cyclization O O O O H O O EtO C C C C C C OEt OEt NaEtO - OEt OEt OEt OEt OEt O O OEt - O O + EtO- cyclic keto ester 97 Other Claisen Type Reactions – There are three types of compounds that can do Claisen type reactions. They are shown below. The main difference between classic Claisen reaction and the ones shown here is the molecule that the Claisen nucleophile attacks. In the classic Claisen reaction the nucleophile attacks another ester, in these reactions the nucleophile attacks something other than an ester. Typically, in these reactions, the nucleophile will attack an alkyl halide like ethylbromide, but it could also attack a ketone or aldehyde. The nucleophiles are made using one of the following compounds. O C2H5 O O C C2H5 O CH2 C2H5 O C H3C C CH2 O CH2 H3C O H3C O O dione Acetoacetic Ester Malonic Ester Malonic Ester Synthesis – makes substituted acetic acids (the acid is circled) O C2H5 O CH2 C2H5 O O O C NaNH 2 C C2H5 O C CH- C2H5 O C-C-Cl O 2 C2H5OH + CO2 C + CH C ( SN2 ) C2H5 C O C2H5 O H , H2O, Heat H2C C spont. decarbox. HO C C C C O O O C C Acetoacetic Ester Synthesis – makes substituted acetones (the acetone is circled) O C2H5 O C CH2 H3C O O NaNH 2 C C2H5 O C CH- H3C C-C-Cl C2H5 C2H5OH + CO2 C + CH C ( SN2 ) H3C C O O H , H2O, Heat H3C C C O O O H2C C spont. decarbox. Dione O H3C CH2 H3C C NaNH 2 H3C C CH- H3C O O O C C-C-Cl H3C CH C ( SN2 ) H3C C C C O O 98 C H+, H2O, Heat No Reaction - the product is not a keto acid. Aldol Reactions Between two Aldehyde/Ketones Classic Aldol – two of the same aldehydes or ketones O C O H C C H 1/2 eq. NaNH2 or ButLi, or NaEtO C C _ C C H C _ H + H H O C O C C C C C H C O H C C C C H C H2O O C OH Aldol Crossed Aldol – two different aldehydes or ketones O C O H C 1 eq. NaNH2 H or ButLi, or NaEtO C C C C _ C H C _ H + C H H O O C C C C C H C C O H C C C C H C H2O O C C OH C Aldol Cyclization O H3C C O C C C O CH3 CH2- O O O NaNH 2 C O- O O H2O If you have excess base present then all of these aldol products can undergo dehydration by the following mechanism; C O H C C C C H C O OH C C _ C C C H C O OH C C C C H Base H O H H OH O H _ OH 99 O C C OH Exam 3 – Practice Synthesis Problems Please synthesize each of the following compounds given the starting reactant shown. You may use any other carbon compounds or inorganic reagents you need to accomplish the synthesis. O C C C Br C C C O C O C C O C C C C C C N C C H O C C O C O O C C C C C C C OCH3 CH2CH2NH2 CH3Br O O H3C O O C OH O O C OH CH3 Aspirin O C O O C C O O C C C 100 C C C Exam 3 – Practice Synthesis Answers C C C O C C O 1. HCN 2. H+, H2O, Heat 3. SOCl2 4. Acetic Acid Br 1. NH2-C2H5 2. LiAlH4 C C C C C C C C O O N C C C C H C C C C O O O C O 1. Br2, light 2. tButO3. NBS 4. KCN 5. BH3, H2O2, NaOH, Water 6. H+, Water, heat C OCH3 1. NaNH2 2. Ethyl Acetate 3. H2O, H+, Heat C C C C CH2CH2NH2 CH3Br 1. KCN 2. LiAlH4 O O H3C 1. Br2, PBr3 2. KOH, Ether 3. K2Cr2O7, H2SO4 4. HO-C-C-OH, H2SO4 C OH O O O O C 1. CH3Cl, AlCl3 2. HSO3F, H2O2 3. KMnO4, H+, H2O, HAc, H2SO4 OH CH3 O C Aspirin O O O 1. NaNH2 C C O C C C O 2. C C C C 3. H+, Water, Heat 101 C C C Chem 241 Exam #3 – Aldehydes/Ketones Name___________________ Closed Book Exam - No books or notes allowed. All work must be shown for full credit. Question 1(18 ) Credit 2(32) 3(16) 4(14) 5(20) TOTAL 1) Please name the structure of the following compounds. H O C O C C O C C C H O O C C C O H3C 102 O O C C CH3 2) Please provide the product of each of the following reactions; O C C C HOCH 2CH 2OH, H+ O C C C 1. HCN 2. H+, H2O, Heat O C C C NaBH4, H2O O C C C N2H4 O C C H 1. Ag(NH3)2+, KOH 2. H+, H2O O C C C Br2, NaOH O C C H CrO3, H2SO4 C C O3, (CH 3)2S C C C C C C O KOH, H2O C H O C C Cl 1. C-C-MgBr 2. H2O, H+ 103 3) Please show the complete mechanism of the formation of a Schiff base using acetone (propanone) and ethyl amine (ethanamine). 4) Please show the complete mechanism of the formation of an acetal using acetone and ethanol. 5a) Please describe why aldehydes are generally more reactive than ketones. 5b) Please describe why aldehydes and ketones go through nucleophilic addition reactions and acids tend to go through nucleophilic acyl substitions. 104 6) Using benzene or alkyl halides that are two carbons or less, synthesize each of the following compounds. Note: CO2, and CO are not organic compounds and may also be used. H O C O C C C H O O O O C C OH C C C 105 Chem 241 Exam #3 – Aldehydes/Ketones Name___Answer Key ____ Closed Book Exam - No books or notes allowed. All work must be shown for full credit. Question 1(18 ) Credit 2(32) 3(16) 4(14) 5(20) TOTAL 1) Please name the structure of the following compounds. H O C O C C O C C C H Benzaldehyde 4-oxo-pentanal O O C C C Acetone benzophenone or diphenylmethanal O H3C Cyclopentanone O O C C CH3 2,3-butadione 106 2) Please provide the product of each of the following reactions; O C O C C HOCH 2CH 2OH, H+ O O C C C C 1. HCN 2. H+, H2O, Heat HO C COOH C O C OH C C NaBH4, H2O C C C NH2 O C N C C N2H4 C C O O C C H Ag(NH3)2+, 1. 2. H+, H2O KOH C C O C C Br2, NaOH C C O H CrO3, H2SO4 C O3, (CH 3)2S C C C C C C O C O C C KOH, H2O C H C C Cl C C O and C OH O O C OH H O C C H C C OH O C C OH O C C C 1. C-C-MgBr 2. H2O, H+ C 107 C C C C C H C C C H OH 3) Please show the complete mechanism of the formation of a Schiff base using acetone (propanone) and ethyl amine (ethanamine). O O H H O + C C H C C C C C C C H2N-C- C O H C C C H N C C H H C H2O + C C N C C C H C O H C C N C H+ + C C H C C N C O H C C N C C H C 4) Please show the complete mechanism of the formation of an acetal using acetone and ethanol. O O H H O + C C H C C C C C H H2O + C C C O C O H C C C H O C O H C C O C HO-C-C C C C O H C C O C C H+ + C C C C HO-C-C H C O C C C O C C C C O C C C O C C + H+ C 5a) Please describe why aldehydes are generally more reactive than ketones. Aldehydes are more reactive than ketones because they have only one methyl group pushing electrons into them rather than two. Ketones have more methyl groups and this reduces the partial charge on the carbonyl carbon making them less reactive than aldehydes. 5b) Please describe why aldehydes and ketones go through nucleophilic addition reactions and acids tend to go through nucleophilic acyl substitutions. Aldehydes and ketones do not have good leaving groups therefore they undergo addition reactions that break the double bonded oxygen. Acids have good leaving groups. This allows them to undergo substitution and preserve the carbonyl carbon (the double bonded oxygen). 108 6) Using benzene or alkyl halides that are two carbons or less, synthesize each of the following compounds. Note: CO2, and CO are not organic compounds and may also be used. H H O C O C 1. CH3Cl, AlCl3 2. NBS 3. KOH, Ether 4. PCC O C C C C C Br 1. Mg, dry ether 2. CO C 3. H+, H2O O C H C H O O 1. Br2, AlBr3 2. Mg, dry ether 3. CO2 4. H+, H2O 5. SOCl2 6. Benzene, AlCl3 O O Br O C C OH C C C C Br C C C 1. KOH, ether x 2 2. CO, H+, heat Br 1. Mg, Dry Ether 2. CO 3. Zn(Hg), HCl 4. Br2, h C 5. KOH, ether 6. PCC O O C C O C C Br 1. KOH 2. PCC C C H 109 1. 1 eq NaNH2 2. C-C=O 3. H+, H2O C C OH C C C Chem 241 Exam #3 – Acids/Derivatives Name___________________ April 26,1999 Closed Book Exam - No books or notes allowed. All work must be shown for full credit. Question 1(18 ) Credit 2(32) 3(16) 4(14) 5(20) TOTAL 1) Please name the structure of the following compounds. O N C O C C N C C O O O C H O C C C N C C C O C Cl Br C C C O O C C OH 110 C OH O 2) Please give the product of each of the following reactions. If the given reaction does not occur, write No Rxn. O C C C C2H5NH 2 OH O C C C OH Cl 1) PBr3, Br2 2) H2O, H+ 1) HCN 2) H+, H2O, Heat 3) C2H5OH, H2SO4, H2O O C C H2SO4, Heat C OH O C C C P 2O 5 NH2 O C C 1. Ag(NH3)2+, KOH 2. H+, H2O C H O HO C C C C H2SO4, Heat C OH Br C C C H3C C 1. Mg, Ether 2. CO2 3. H+, H2O CH2 KMnO 4, H+ H2O, Heat 111 3a) Please draw the mechanism of the Hell-Volhardt-Zelinsky reaction using propanoic acid. 3b) Please draw the mechanism of the hydrolysis of a nitrile into an acid in base. 4a) Please explain why we almost always do nucleophilic substitutions on acid chlorides, anhydrides, and esters, but rarely on acids or amides. This is not “a better leaving group” question! 4b) Please rank the following acids in order of increasing acidity (1 is lowest). Please explain your reasoning. O C C C C CH3 C OH C C Br O C C OH C C C C OH 112 Br O C O C C OH 5) Starting with benzene or alkyl halides of two carbons or less synthesize each of the following compounds. COOH N O O O O O C C O C O C H O C C C C N 113 Chem 241 Exam #3 – Acids/Derivatives Name___________________ April 26,1999 Closed Book Exam - No books or notes allowed. All work must be shown for full credit. Question 1(18 ) Credit 2(32) 3(16) 4(14) 5(20) TOTAL 1) Please name the structure of the following compounds. O N O 5-aminopentoic acid lactam C C C N C C O O O C H cyclobutyl methanoate butadioic anhydride O C 3-methylbutanitrile C C N C C C N-propylpropanamide O C Cl Br C C C O O C C OH benzoyl chloride O 2-bromobutanoic acid 114 C OH acetic acid 2) Please give the product of each of the following reactions. If the given reaction does not occur, write No Rxn. O C C C O C2H5NH 2 C C C OH N C C H O C C C OH Cl Br 1) PBr3, Br2 C O C C 2) H2O, H+ OH O C 1) HCN 2) H+, H2O, Heat O C C 3) C2H5OH, H2SO4, H2O O O C C H2SO4, Heat C C O C C C OH O O C C C P2O5 C NH2 C C O O C C Ag(NH3)2+, 1. 2. H+, H2O C N KOH C C C OH H O O HO C C C C H2SO4, Heat C O OH Br C C COOH C H3C C 1. Mg, Ether 2. CO2 3. H+, H2O C C C COOH CH2 KMnO 4, H+ H2O, Heat 115 C C C 3a) Please draw the mechanism of the Hell-Volhardt-Zelinsky reaction using propanoic acid. O O C C C PBr3 OH C C C O P H Br OH C C C O Br C Br C Br O + Br- P Br H OH Br2 C Br O Br keto-enol C (Br+ + Br-) C C Br C C C Br O P Br H O OH C C C C Br C Br + C H O Br C Br C C Br + H+ Br 3b) Please draw the mechanism of the hydrolysis of a nitrile into an acid in base. OH C C OH- N C OH H2O N C C C H N OH OH- C H C N OH H2O O O NH2- + C H2O + C C OH C H OH- C N C H OH H2O OH H N H OH NH3 + OH- 4a) Please explain why we almost always do nucleophilic substitutions on acid chlorides, anhydrides, and esters, but rarely on acids or amides. This is not “a better leaving group” question! Acids and amides have OH and NH2 groups that will react with and neutralize any incoming nucleophile. The other compounds mentioned do not have any OH’s or NH’s so they do not suffer from this problem 4b) Please rank the following acids in order of increasing acidity (1 is lowest). Please explain your reasoning. O C C C C CH3 C C C OH Br O C C C C OH Br O C C C O C OH C OH Methyl groups push in electrons making acids weaker so #1 is the weakest. The acid with no groups is next, and then as the halogen gets closer to the acid group, the acid gets stronger. 116 5) Starting with benzene or alkyl halides of two carbons or less synthesize each of the following compounds. O COOH Br N C O O C O Br C C C OH KMnO 4, H+ H2O, Heat N O 1. tButO-, tButOH 2. NBS Br 3. NaCN, THF 4. HBr, PBA C O C 1. KOH, Ether 2. KMnO4, H+, H2O, Heat 3. SOCl2 4. Benzene, AlCl3 CH3CH3Br O CH3 C 1. tButO-, tButOH 2. NBS 3. NaCN, THF NC 4. HBr, PBA 5. NaCN, THF C C C CN 1. NH3(liq) 2. H+, H2O, Heat 3. Heat C C C CN 1. H+, H2O, Heat 2. H2SO4, Heat O O O O C C C Br O C C C O O C H 1. KOH, Ether 2. KMnO4, H+, H2O, Heat C C O C O OH 1. KOH, Ether CH3Br 2. PCC HO C H2SO4, Heat C C C O O C H H 3. Tollens Reagent Br O O C C C C C C Br C N 1. NaCN, THF 2. H+, H2O, Heat 3. HVZ 4. NH3 C C C O NH2 1. KOH, Ether 2. PCC 3. P2O5 C C C C N Note: I used Tollens reagent on the 4th synthesis because most oxidations will take formaldehyde and convert it all the way to CO2. Tollens will only take the oxidation to the acid, not CO2. 117 Final Exam 118 Protein Sequencing Problems 1) A small peptide was completely hydrolyzed to yield the following set of amino acids; Ala + Arg + Gly + 2 Met + Lys + Ser + Val In addition the peptide was subject to the following tests with the results given. Sanger Reagent - DNP Gly and ,-DNP Lys Carboxypeptidase A - a) Ser b) All the rest Cyanobromide - a) Ala + Gly + Lys + Met b) Met + Val c) Arg + Ser Chymotrypsin - Nothing Released Trypsin - a) Ala + Gly + Lys b) Arg + 2 Met + Val c) Ser What is the order of amino acids in the peptide chain? 2) A small protein was completely hydrolyzed and found the contain Arg + Cys + 2 Lys + Met + Thr + Val. Based on the observations below, reconstruct the protein sequence. FDNB - DNP-Arg released Carboxypeptidase A a) Val b) All the rest Carboxypeptidase B Nothing released Cyanobromide - a) Lys-Cys-Met-Arg b) Thr-Val-Lys Trypsin - a) Arg-Lys b) Cys-Thr- Lys-Met c) Val Are there any unresolved amino acids? Which are they? 119 Answer Key Problem #1 Sanger – Gly must have been the AA on the far left side (amino side) of the protein sequence. Lys has an NH2 on its side chain. That is why FDNB attacked it. Chain = Gly ~ Carb A – Carb A attacks the AA on the other end of the protein (the carboxylic acid side). Since Ser was released, it must have been the last AA in the chain. Chain = Gly ~~~~~~~~~~~~~~~~~~~~~~~~~~Ser Cyanobromide – This cleaves on the carboxyl side of Met. This means that anything attached to the NH2 side of Met remains attached. There from each piece we deduce, a) Gly is the first AA in the chain - Gly~(Ala~Lys)~ Met or Gly~( Lys~Ala)~ Met b) Val~Met in this order because Val is attached to Met’s NH2 c) Ser is the last AA so must be Arg~Ser Chain = Gly ~(Ala~Lys)~Met~Val~Met~Arg~Ser or Gly ~(Lys~Ala)~Met~Val~Met~Arg~Ser Trypsin – All we need to do is to resolve the position of Ala with respect to Lys. The first piece cleaved off does this for us. If the order had been Gly~Lys~Ala~ then trypsin would have cleaved between Lys and Ala and you would have gotten a piece containing just Gly+Lys, but you didn’t, you got Gly+Lys+Ala which means that the order must have been Gly~Ala~Lys. So the order of the protein must have been, Final Chain = Gly~Ala~Lys~Met~Val~Met~Arg~Ser 120 Problem #2 Moving a little faster this time, Sanger/CarbA&B – Gives us the following information Chain = Arg~~~~~~~~~~~~~~Val Cyano – Cleaves Met on the right, so a) Arg~(Lys~Cys)~Met or Arg~(Cys~Lys)~Met b) (Thr~Lys)~Val or (Lys~Thr)~Val Chain = Arg~(Lys~Cys)~Met~(Thr~Lys)~Val Trypsin – Cleaves Lys and Arg on the right, so a) Arg~Lys was released, but since we also know Arg~(Lys~Cys)~Met from above, then the order must be Arg~Lys~Cys~Met b&c) Since Val was released separately, Lys must have been attached to it, therefore since we knew from Cyano (b) (Thr~Lys)~Val or (Lys~Thr)~Val, the order must have been Thr~Lys~Val. Final Chain = Arg~Lys~Cys~Met~Thr~Lys~Val There are no unresolved amino acids 121 Carbohydrate Problem Set 1) Galactose is the C4 epimer of glucose. Please draw lactose, which is a disaccharide, made by bonding galactose to glucose using a 1,4’ link. Both sugars must be cyclic. 2) Please draw the ring structure of the C2 epimer of galactose. 3) Please draw the C3 epimer of glucose. 4) Please draw the ring structure of Talose. 5) Please draw the ring structure of Fructose. 6) What is a reducing sugar? Why is it reducing? 7) How do we determine whether a structure is D or L? Is R 3-hydroxybutanoic acid, D or L? 8) Draw lactose. It is galactose––1,4’ glucose. 9) Draw gentiobiose. It is glucose--1,6’ glucose 10) Show the complete mechanism for the base conversion of fructose into glucose. 11) Show the complete mechanism for the acid conversion of fructose into glucose. 122 Carbohydrate Problem Set 1) Galactose is the C4 epimer of glucose. Please draw lactose, which is a disaccharide, made by bonding galactose to glucose using a 1,4’ link. Both sugars must be cyclic. H O H O OH HO CH2OH CH2OH C O OH H H OH H OH O OH OH OH OH OH Glucose CH2OH Galactose Glucose 2) Please draw the ring structure of the C2 epimer of galactose. CH2OH HO CH2OH HO O OH O OH OH OH OH OH Galactose Gulose 3) Please draw the C3 epimer of glucose. CH2OH CH2OH O OH O OH OH HO OH OH Glucose OH OH Allose 4) Please draw the ring structure of Talose. CH2OH HO O OH OH OH Talose 123 5) Please draw the ring structure of Fructose. HOH2C O HO CH2OH OH OH 6) What is a reducing sugar? Why is it reducing? A reducing sugar causes the reduction of other things by becoming oxidized. This occurs aldehyde end of the sugar. Aldoses are reducing, ketoses are not. When oxidized, aldoses become acids. 7) How do we determine whether a structure is D or L? Is R 3-hydroxybutanoic acid, D or L? When drawn in a Fisher projection, a D molecule has the second to last OH group on the right side of the molecule. An L molecule has the second to last OH on the left. HO O H C H C This is R 3-hydroxybutanoic acid. The arrow indicates the rotation. You will note that the arrow is going counter-clockwise, but because the low priority hydrogen is on the H right, we are seeing the rotation backwards so the actual rotation is clockwise (R). Since OH the second to last carbon has the OH on the right, this molecule is D. H C H C H 8) Draw lactose. It is galactose––1,4’ glucose. CH2OH HO O OH O OH OH CH2OH CH2OH HO O OH O OH OH CH2OH OH O OH OH Galactose OH OH Glucose OH Galactose 1,4'- glucose 9) Draw gentiobiose. It is glucose--1,6’ glucose CH2OH CH2OH O OH OH O OH OH OH OH O O CH2 OH OH Glucose CH2OH O OH OH OH OH Glucose OH OH Glucose 1,6' glucose 124 OH 10) Show the complete mechanism for the base conversion of fructose into glucose. H H _ C HO H OH C O C H C OH H C OH H C OH C OH C H _ OH- HO C O C H HO C O C H H2O HO OH- H H C OH- + O H C OH HO C H H2O _ HO C O C OH C H H HO C O- C OH C H 11) Show the complete mechanism for the acid conversion of fructose into glucose. H H C C H OH H+ H O C C H OH + O C OH C OH HO C H HO C H HO C H _ + H+ H HO H H+ + C O H C OH HO C H 125 H H C O+ C OH C H H H C O+ H C OH HO C H Chem 241 Final Exam Name___________________ May 27, 2000 CLOSED BOOK EXAM - No books or notes allowed. ALL work must be shown for full credit. You may use a calculator. Question 1(10) 2(10) 3(8) 4(16) 5(15) 6(20) Total Credit Question 7(15) 8(12) 9(12) 10(42) 11(10) 12(30) Total Credit 1a) Please draw the complex splitting diagram for carbon B in the following compound. Where will the signal be centered? Jab = 11 Hz and Jbc = 9 Hz Br C C C C A B C Cl 1b) What is the possible explanation for the differences in the postion of the aromatic protons in the following compounds? Benzene = 7.37 Toluene = 7.17 p Xylene = 7.05 126 2) Approximately where would you expect to find a C=S bond in an I.R. spectrum? Show your work and state your assumptions. C= 12, S=32, O=16. Your answer should be somewhere between 1000 and 3000. I want and exact anwser. Show your math. 3) Please explain why we almost always do nucleophilic substitutions on acid chlorides, anhydrides, and esters, but rarely on acids nor amides. This is not “a better leaving group” question! 4a) One of the products below was made using a Michael reaction and the other was made using a Claisen reaction. Which is which? C C C O C C C C O C C C C O O 4b) For the Michael product given above, show the Michael reaction that made it. Just show the two (or more) reactants and the product(s). Do not give a mechanism. 127 5a) Please draw all of the resonance structures for ortho attack of aniline with the nitro cation (NO2+). You do not have to make the nitro cation. 5b) Please draw the complete mechanism for the Friedel-Craft alkylation of benzene using bromobutane as the alkylating agent. 5c) Please give the complete mechanism for the base hydrolysis of phthalic anhydride. O O NaOH H2O O 6) Please interpret and draw any two of the three compounds given on the back pages based on their IR and NMR spectra. 128 7a) Please draw glycine (R = H) dissolved in a neutral solution. 7b) What are the four levels of protein structure? Describe each of them. a) b) c) d) 8) Galactose is the C4 epimer of glucose. Please draw lactose, which is a disaccharide, made by bonding galactose to glucose using a 1,4’ link. Both sugars must be cyclic. 9) Please circle the terpene units found in the following compound. CH3 CH3 CH3 CH3 129 10) Please give the product for each of the following reactions. Cl HNO 3, H2SO4 C NH2 O KOH, H 2O O NO2 AlCl3, C C C Cl O CH3 C CH3 1/2 eq. NaEtO Water O CH3 C NH2 P2O5 O O 1. NaEtO O Et 2. H+, H2O, O NH2 H2SO4, OH O O O O 1. 2x NaEtO 2. Br-C-C-C-C-Br 3. H+, H2O, 130 10 cont’d) 1. C-C-C-Cl, AlCl3 2. NBS 3. KCN 4. H+, H2O, NH2 C COOH 1. LiAlH4 2. H2SO4 O O O O O NaEtO, 1. C-C-MgBr 2. Water O O HO-C-C- OH, H2SO4 O C C 1. PBr3, Br2 C OH 2. H2O, H2SO4 11) Synthesize one of the compounds given below starting with alcohols, alkyl halides or alkanes of three carbons or less. You cannnot start with benzene. O N H2N O CH C OH O CH OH O CH3 NH2 131 12a) Please draw the product of the following Diels-Alder reactions; O O O O 2x 12b) Please draw the product and the MO diagram for a 6+4 reaction. Show the HOMO, LUMO, and state whether the reaction occurs thermally or photochemically. 12c) What are the rules of aromaticity? 12d) What is the “endo” rule? 132 Chem 241 Final Exam Name_Answer Key________ May 27, 2000 CLOSED BOOK EXAM - No books or notes allowed. ALL work must be shown for full credit. You may use a calculator. Question 1(10) 2(10) 3(8) 4(16) 5(15) 6(20) Total Credit Question 7(15) 8(12) 9(12) 10(42) 11(10) 12(30) Total Credit 1a) Please draw the complex splitting diagram for carbon B in the following compound. Where will the signal be centered? Jab = 11 Hz and Jbc = 9 Hz 2.3 Br C C C C A B C Cl 1b) What is the possible explanation for the differences in the postion of the aromatic protons in the following compounds? Benzene = 7.37 Toluene = 7.17 p Xylene = 7.05 Methyl groups push electrons into the things to which they are attached. Pushing electrons into a benzene ring causes the benzene’s hydrogens to become shielded and less downfield that benzene alone. The more methyls you have, the more shielded benzene become and the less downfield are the benzenes hydrogens. 133 2) Approximately where would you expect to find a C=S bond in an I.R. spectrum? Show your work and state your assumptions. C= 12, S=32, O=16. Your answer should be somewhere between 1000 and 3000. I want an exact anwser. Show your math. Since both of them have double bonds, assume k1 = k2 which means that they will cancel, so the only difference is the mass effect (). Since C=O is at 1710 cm-1 use it as 1. 12 32 1710 12 32 v2 1516cm 1 12 16 v2 12 16 k1 2 k2 1 v1 v2 3) Please explain why we almost always do nucleophilic substitutions on acid chlorides, anhydrides, and esters, but rarely on acids nor amides. This is not “a better leaving group” question! Both acids and amides have H’s available because of the hydrogen bonding of the OH and NH2. These acidic protons interfere with nucleophilic substitutions by neutralizing the nucleophile. That is why acid derivatives other than acids and amides are used in most syntheses requiring acids. 4a) One of the products below was made using a Michael reaction and the other was made using a Claisen reaction. Which is which? C C C O C C C C O Michael C C C C O O Claisen 4b) For the Michael product given above, show the Michael reaction that made it. Just show the two (or more) reactants and the product(s). Do not give a mechanism. O Et 1, NaNH2 2. C=C-C=O 3. H+, H2O, O O O O 134 5a) Please draw all of the resonance structures for ortho attack of aniline with the nitro cation (NO2+). You do not have to make the nitro cation. + NH2 NO2 A NH2 NH2 NH2 NH2 A NO2+ + NO2 NO2 NO2 B B + + 5b) Please draw the complete mechanism for the Friedel-Craft alkylation of benzene using bromobutane as the alkylating agent. Br + AlBr3 H - Br AlBr3 + + AlBr4Hydride Shift H + + + H+ 5c) Please give the complete mechanism for the base hydrolysis of phthalic anhydride. O O- O OH O O OH - OH O O- O H2O O O OH + OH- OH O 6) Please interpret and draw any two of the three compounds given on the back pages based on their IR and NMR spectra. 135 7a) Please draw glycine (R = H) dissolved in a neutral solution. H + H3N O C C O- H 7b) What are the four levels of protein structure? Describe each of them. a) Primary – the simple sequence of proteins b) Secondary – folded into helix or sheets c) Tertiary – Helix or sheets folded into globular structures d) Quaternary – Two or more globular structures bonded together 8) Galactose is the C4 epimer of glucose. Please draw lactose, which is a disaccharide, made by bonding galactose to glucose using a 1,4’ link. Both sugars must be cyclic. H O C H OHOH OH HO H H OH H OH H OH H O H HO H H H O O OH H CH2OH HO H H Glucose 9) Please circle the terpene units found in the following compound. CH3 CH3 CH3 CH3 136 H OH OH 10) Please give the product for each of the following reactions. Cl Cl HNO 3, H2SO4 NO2 C NH2 O OH C O KOH, H 2O C OH O NO2 AlCl3, C C C Cl No Reaction - The ring is deactivated O O CH3 C CH3 CH3 1/2 eq. NaEtO CH3 Water C C CH3 O CH3 C C NH2 P2O5 CH3 C N O O O 1. NaEtO O Et 2. H+, H2O, O O NH2 O H2SO4, N OH O O H O O 1. 2x NaEtO 2. Br-C-C-C-C-Br 3. H+, H2O, O HO 137 OH 10 cont’d) COOH 1. C-C-C-Cl, AlCl3 H3C C CH3 2. NBS 3. KCN 4. H+, H2O, NH2 C COOH NH2 C C OH 1. LiAlH4 2. H2SO4 O O O O O NaEtO, O O O O 1. C-C-MgBr 2. Water O O HO-C-C- OH, H2SO4 O Br O C C 1. PBr3, Br2 C OH O C O C C 2. H2O, H2SO4 OH 11) Synthesize one of the compounds given below starting with alcohols, alkyl halides or alkanes of three carbons or less. You cannnot start with benzene. O N H2N O CH C OH O CH OH O CH3 NH2 See last page 138 12a) Please draw the product of the following Diels-Alder reactions; O O O O O O O O 2x 12b) Please draw the product and the MO diagram for a 6+4 reaction. Show the HOMO, LUMO, and state whether the reaction occurs thermally or photochemically. + h LUMO + - + + + - + - + + Zero of Ene rgy Reaction occurs photochemically HOMO + + + - + - + + + + 12c) What are the rules of aromaticity? 1. Completely conjugated ring 2. Flat 3. No orbitals on zero of energy 12d) What is the “endo” rule? A molecule is endo with the dienophile is oriented in such a way so that the electron withdrawing groups is able to interact with the p orbitals of the diene. 139 Answers to syntheses question Br C C O 1. t-ButO-, tButOH 2. NBS Br 3. CN4. HBr, PBA C C C C CN 1. NH3 2. H+, H2O, H2N C C C C OH O H H2SO4, Heat N OH C PCC C OH O C C 1. 1/2 eq. NaNH2 2. H2O C C C 1. SOCl2 2. KMnO4, H+ H2O, Heat C O H2N CH C OH OH Br NH 3 C CH OH C C Cl O OH O PBr3, Br2 C C C C H C H H Br C C C 1. t-ButO-, tButOH 2. NBS 3. Mg, dry ether H C C O HO-C-C-C-OH H2SO4, Heat C C C 1. NBS x 2 2. t-ButOt-ButOH KOH, Ether C C C C C OH H2SO4 Heat (E1) C Br C O C C C NH2 NO2 OH O 1. CH3OH, PCC 2. H2SO4 MgBr C 1. C-C-COCl 2. AlCl3 3. con. H2SO4 con. HNO3 C OH O H C C CH3 PCC C O OH OH C O Zn(Hg), HCl O 140 Cl C t-ButOt-ButOH 1. KMnO4, H+, H2O, Heat C 2. SOCl2 C C C C OH Chem 241 Final Exam Name________________________ May 23, 2005 Closed Book Exam - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(21) 2(20) 3(24) 4(20) 5(15) Total Credit Question 6(20) 7(30) 8(15) 9(15) 10(20) Total Credit 1) Please name the following compounds. O O O C C NH2 H C N H HO Cl C C C C N C HO C OH O O C 141 C C C N 2a) Please draw the mechanism of the acid cleavage of acetic anhydride using H2SO4 and water. 2b) Please draw the mechanism of the Hell-Volhardt-Zelinsky reaction using butanoic acid, PBr3, and Br2 2c) Please draw the mechanism for the Claisen reaction that occurs when ethyl acetate reacts with half an equivalent of NaNH2. 2d) Please draw the mechanism for the formation of an enone using acetone, a strong base, and water (if necessary). 142 3a) Please draw all of the resonance structures for ortho attack of aniline with a chloronium ion (Cl+). 3b) Please draw the complete mechanism for the hydroxylation of benzaldehyde. 3c) For each of the following compounds label them as either an activator or deactivator, and as an ortho, para, or meta director. NHCH3 CN H3C C Act/Deact _______ ________ ________ Director _______ ________ ________ 143 CH3 4a) Using drawings and words, please show how a quartet is formed in NMR spectroscopy. 4b) What is the relative height of each peak in a hextet? 4c) If Jab = 8 and Jbc = 7, what is the complex splitting diagram for the following molecule?. H H a C O b H c C C H H Cl 5) Please draw the MO diagram for a 6 +2 reaction. Label the HOMO, LUMO, the orbital symmetry and state how the reaction occurs. 6) Please interpret and draw any two of the three compounds given on the back pages based on their IR and NMR spectra. 144 7) Please give the product for each of the following reactions. 1. HCN C C C Cl 2. LiAlH4 O C C C O C O C C C2H5NH2 1. NaNH2 C CH3 2. Benzaldehyde 3. H2O O 1. C-C-C-C-Br N 2. N2H4, Heat O O C O C C C 1. NaNH2 C O C C C C NH2 2. H2O Heat HO H C O AlCl3, CH3Cl O N2H4, OH-,heat Cl OH 1. PCC 2. C2H5MgBr 3.Water, H+ 145 8a) Draw a phospholipid and a fat. Label each of them. You can use squiggly lines for long chains. 8b) What is a soap and how do they work? Please draw a picture as part of your answer. 8c) Most candles are made from a compound that, though called a wax, is not really a wax. What is it and how does it differ from a true wax? 146 9a) What are Hückel’s Rules? 9b) An OH peak in NMR is usually a singlet, why? And when would you expect it to be a triplet? 9c) What are the restrictions on Friedel Craft Alkylation? 10) Starting with alkanes, alcohols, benzene, or cyclopentane, synthesize two of the following compounds. COOH N H C O NH2 147 O Chem 241 Final Exam Name__Answer Key______ May 23, 2005 Closed Book Exam - No books or notes allowed. All work must be shown for full credit. You may use a calculator. Question 1(21) 2(20) 3(24) 4(20) 5(15) Total Credit Question 6(20) 7(30) 8(15) 9(15) 10(20) Total Credit I want to drop my lowest exam _____________________. Signature 1) Please name the following compounds. O O O C C NH2 H C N H HO methanal (formaldehyde) 2-aminoacetic acid 5-aminopentanioic acid lactam Cl C C C C N C HO C OH N,N,N-triethylamine 4-chloro-2-hydroxyphenol O O C C C C N diphenylmethanone (benzophenone) N,N-diphenylbutanimide 148 2a) Please draw the mechanism of the acid cleavage of acetic anhydride using H2SO4 and water. O O O + +O H C C O O H C C H C C O C C C O C C O + C C O O H O C C H O H O H O+ C OH + HO C C H+ C C O O H C C O H C O + O C C HO O O H C C +O H H O C H2O C + H+ C 2b) Please draw the mechanism of the Hell-Volhardt-Zelinsky reaction using butanoic acid, PBr3, and Br2 Br O O O C C C PBr3 C C C C + C OH - O P H Br C Br C C + C O P H Br Br - Br OH C C C C Br O O keto-enol C - C C C C Br C C + C O P Br H Br Br + H+ Br Br+ OH C C C C Br C C +O H C Br C Br + O C C Br C C Br 2c) Please draw the mechanism for the Claisen reaction that occurs when ethyl acetate reacts with half an equivalent of NaNH2. O C C O C CH3 NaNH 2 O C C + C O O O C C O- C C O CH2- + C C O C C C O CH3 C C O C O C CH3 OH2 C C O C C CH3 2d) Please draw the mechanism for the formation of an enone using acetone, a strong base, and water (if necessary). O H3C O H3C C CH3 O C CH3 NaNH 2 H3C C - O H3C CH2 C CH3 C C O- CH3 H2O O H3C C + OH- O CH3 C H H3C C CH3 C - CH3 C C H CH3 149 OH NaNH 2 H3C O H CH3 C C C H CH3 OH + OH- 3a) Please draw all of the resonance structures for ortho attack of aniline with a chloronium ion (Cl+). + NH2 Cl A NH2 NH2 NH2 NH2 A Cl + Cl+ Cl Cl B B + + 3b) Please draw the complete mechanism for the hydroxylation of benzaldehyde. H H H O O H HSO3F H H O O O + OH+ H H O + H2O O OH+ + H+ OH + OH H 3c) For each of the following compounds label them as either an activator or deactivator, and as an ortho, para, or meta director. NHCH3 CN H3C C Act/Deact __Act___ _Deact_ __Act___ Director __o/p___ __m_____ ___o/p__ 150 CH3 4a) Using drawings and words, please show how a quartet is formed in NMR spectroscopy. CH If a hydrogen or set of hydrogens is sitting next to a CH3 group, those hydrogens are split by the combination of spins set up by the methyl group. The hydrogens on the methyl group can have four possible sets of orientations that cause the attached hydrogens to be split into a quartet. CH3 A CH looking at a CH3 will get split into a quartet by the combination of spins produced by the methyl group. 4b) What is the relative height of each peak in a hextet? 1:5:10:10:5:1: 4c) If Jab = 8 and Jbc = 7, what is the complex splitting diagram for the following molecule?. H H a C O b 2.5 H c C C H H Cl 5) Please draw the MO diagram for a 6 +2 reaction. Label the HOMO, LUMO, the orbital symmetry and state how the reaction occurs. +-+-+LUMO + +-++-+ ++-+-- +- ++--++ ++ Zero of Energy HOMO Reaction occurs Photochemically ---+++ h ++++++ 6) Please interpret and draw any two of the three compounds given on the back pages based on their IR and NMR spectra. 151 7) Please give the product for each of the following reactions. 1. HCN C C C C Cl C C NH 2 2. LiAlH4 O O C C C O C O C C C2H5NH2 C C C H N HO CH3 2. Benzaldehyde 3. H2O O 1. C-C-C-C-Br N C C C C 1. NaNH2 C 2. H2O OH O O C C C C NH 2 O O C C 2. N2H4, Heat O O C NH2 Heat N H HO H C O AlCl3, CH3Cl No Reaction O N2H4, OH-,heat Cl Cl OH HO 1. PCC 2. C2H5MgBr 3.Water, H+ 152 C O C 1. NaNH2 C C C C C C 8a) Draw a phospholipid and a fat. Label each of them. You can use squiggly lines for long chains. O O C O C O C O C O C O C C O P C O O C C O O C O Fat (Triglyceride) O O P O O O P O- O Phospholipid 8b) What is a soap and how do they work? Please draw a picture as part of your answer. Soaps are nothing more than the salts of long chain fatty acids. Their tails dissolve into oils and grease which leaves their hydrophilic “heads” poking out into the water. This has the affect of making the surface of the oil look hydrophilic which allows the oil to emulsify in water, which is how soaps remove oils and other dirt from clothing. Fat, oil or dirt Hydrophobic hydrocarbon Hydrophilic acid (head) 8c) Most candles are made from a compound that, though called a wax, is not really a wax. What is it and how does it differ from a true wax? Most candles are made of paraffin which is really just a very long alkane. A true wax, like bees wax, is an ester made from very long fatty acids (~24 carbons) and very long alcohols (~24 carbons). Common waxes are bees wax and carnuba wax, which is the wax used to wax a car. 153 9a) What are Hückel’s Rules? i Completely conjugate ring ii Must be flat iii No orbitals on the zero of energy 9b) An OH peak in NMR is usually a singlet, why? And when would you expect it to be a triplet? The OH in an NMR is a singlet because hydrogen bonding allows the hydrogen to move on and off the oxygen more rapidly that the NMR can “see” it. The hydrogen is not on the oxygen long enough to cause splitting. An OH would be a triplet if the alcohol were in the gas phase. As a gas, the hydrogen is not allowed to leave the oxygen and would therefore be split by neighboring hydrogens. 9c) What are the restrictions on Friedel Craft Alkylation? i The ring cannot be more deactivated that caused by a halogen ii There cannot be any OH or NH2’s on the ring since they will react with the AlCl3 10) Starting with alkanes, alcohols, benzene, or cyclopentane, synthesize two of the following compounds. COOH N H C O NH2 See next page 154 O 1. Br2, h t-ButO-, t-ButOH 3. NBS 4. t-ButO-, t-ButOH Br C C C 1. t-ButO-, t-ButOH 2. NBS 3. KOH, Ether C 4. PCC 1. HBr 2. EtO-, EtOH H C C H C O O C Cl COOH 1. CN2. H+, H2O, Heat 1. FeCl3, Cl2 2. con H2SO4, con HNO3 3. Zn(Hg), HCl NH2 Br C C C 1. t-ButO-, tButOH 2. NBS Br 3. CN4. HBr, PBA O NH2 O C C C CN 1. NH3 2. H+, H2O, H2N C C C C OH O H N 155 H2SO4, Heat

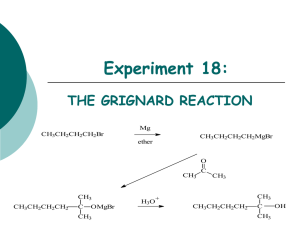


![AL Chem Written Practical (Organic Chemistry) [F.7]](http://s2.studylib.net/store/data/005797652_1-4911d95dd6c8a0840f727bd387aa6027-300x300.png)