Review Chem Equations
advertisement
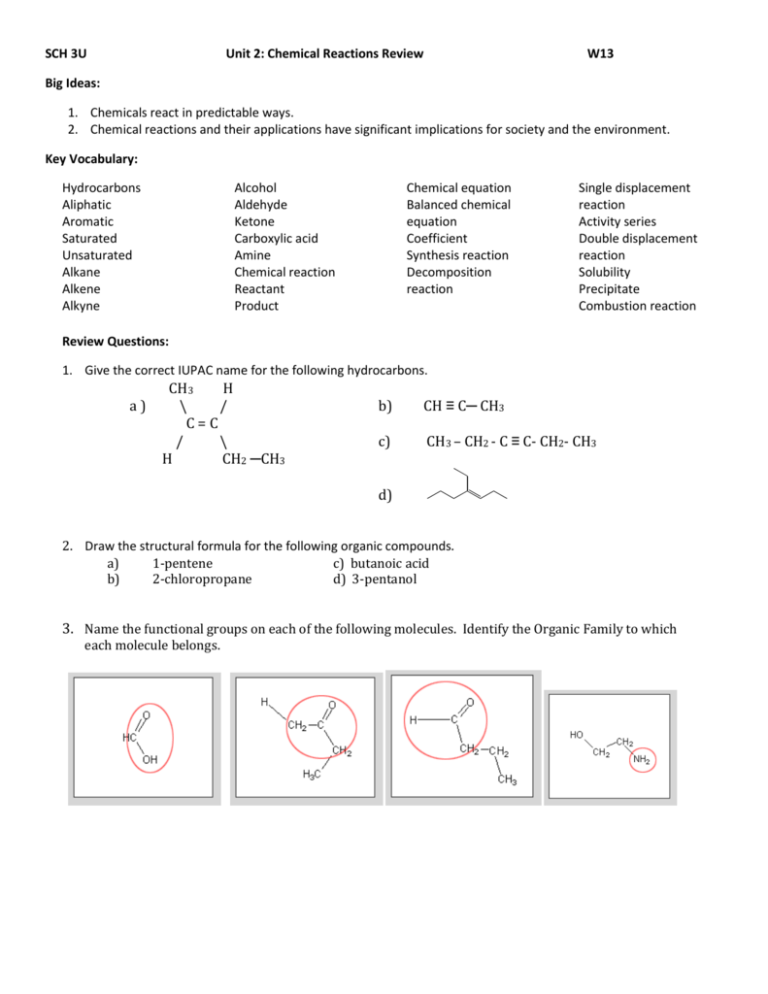
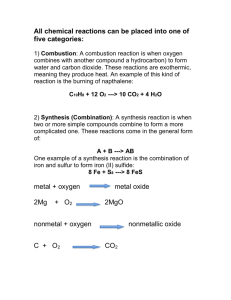
SCH 3U Unit 2: Chemical Reactions Review W13 Big Ideas: 1. Chemicals react in predictable ways. 2. Chemical reactions and their applications have significant implications for society and the environment. Key Vocabulary: Hydrocarbons Aliphatic Aromatic Saturated Unsaturated Alkane Alkene Alkyne Alcohol Aldehyde Ketone Carboxylic acid Amine Chemical reaction Reactant Product Chemical equation Balanced chemical equation Coefficient Synthesis reaction Decomposition reaction Single displacement reaction Activity series Double displacement reaction Solubility Precipitate Combustion reaction Review Questions: 1. Give the correct IUPAC name for the following hydrocarbons. a) CH3 H \ / C=C / \ H CH2 ─CH3 b) CH ≡ C─ CH3 c) CH3 – CH2 - C ≡ C- CH2- CH3 d) 2. Draw the structural formula for the following organic compounds. a) 1-pentene c) butanoic acid b) 2-chloropropane d) 3-pentanol 3. Name the functional groups on each of the following molecules. Identify the Organic Family to which each molecule belongs. 4. For each of the following equations: a. Balance the equation. b. Determine the type of reaction. i. K2SO3 + Sr(NO3)2 + SrSO3 + AgNO ii. Ag + Cu(NO3)2 Cu iii. Fe + S FeS iv. Pb + Mg(CH3COO)2 Pb (CH3COO)2 + v. H2SO4 H2O + SO3 vi. K2SO4 5 H2O K2SO4 + H2O vii. H2SO4 + NaOH H2O + viii. Li3PO4 + CaCl2 LiCl + Ca3(PO4)2 + O2 CO2 + H2O ix. C2H2 5. KNO3 Mg Na2SO4 Predict the products of the following synthesis and decomposition reactions. a) H2 (g) + Cl2 (g) (b) NO2 (g) (c) AuBr3 d) MgO (s) (e) H2O (l) + NO2 (g) (f) SO3 (g) + H2O (l) 6. Determine the products of the following single displacement reactions. Where no reaction is predicted to proceed please write the term ‘NR. a) Au (s) + Pb(NO3)2 (aq) b) Na (s) + AgCl(aq) c) KNO3 (aq) + Fe (s) d) Br2 (l) + KI (aq) e) F2 (g) + KI (aq) f) Li (s) + KI (aq) 7. Predict the potential products of the following double displacement reactions. Write the balanced chemical equation for the following double displacement reactions. Use your solubility table to predict if they will occur. a) aluminum iodide + mercury(II) chloride b) silver nitrate + potassium phosphate c) copper(II) bromide + aluminum chloride d) calcium acetate + sodium carbonate e) ammonium chloride + mercury(I) acetate 8. Several drops of sodium sulfate solution are added to a sample of water to test for contaminants. The possible contaminants in the water are sodium nitrate, calcium chloride, and lead (II) acetate. a) Which of these contaminants might be present if a precipitate forms? b) Write the balanced chemical equation for the reaction that involves this contaminant.