AL Chem Written Practical (Organic Chemistry) [F.7]
advertisement
![AL Chem Written Practical (Organic Chemistry) [F.7]](http://s2.studylib.net/store/data/005797652_1-4911d95dd6c8a0840f727bd387aa6027-768x994.png)
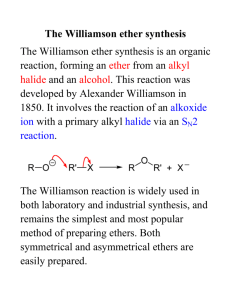
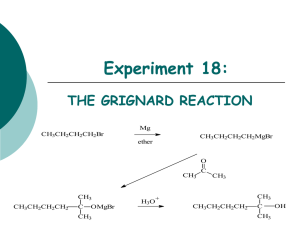
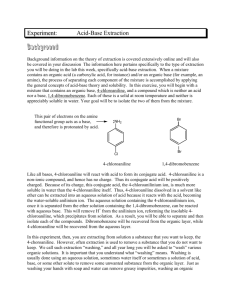
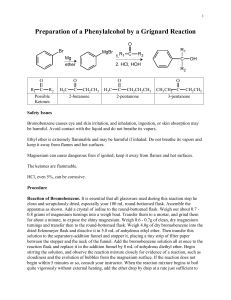
ORGANIC CHEMISTRY AL CHEM REVIEW AL CHEM Written Practical [Organic Chemistry] ~ Organic Synthesis ~ Organic Acid How to separate How to purify Organic Base the product from the product?? rxn mixture ?? (solid/liquid) Others p.1 Common Separation Method Solvent Extraction (use separating funnel) Distillation Fractional Distillation p.2 Common Purification Method Recrystallization Na2SO4 / MgSO4 Drying Decolorization (adding activated charcoal) p.3 1997 P.I Q.8(b) You are provided with a mixture of two liquids, hexan-1-amine (U) and ethyl ethanoate (W). Outline an experimental procedure, based on a solvent extraction process, to enable U to be separated from W. (3M) p.4 Add ether and dilute HCl. *Shake in a separating funnel. The ether layer will contain CH3COOC2H5. Basify the aqueous layer with excess NaOH(aq). and then extract with ether. The ether layer will contain CH3(CH2)5NH2 p.5 1998 P.I Q.8(b) You are provided with a mixture of two liquids, heptanoic acid and hexan-3-one. Outline an experimental procedure, based on a solvent extraction process, to isolate pure heptanoic acid in good yield. (3M) p.6 Add ether and dilute NaOH / Na2CO3 / NaHCO3. *Shake in a separating funnel. Ether layer contains hexan-3-one, aqueous layer contains CH3(CH2)5COO-Na+. Add dilute HCl to aqueous layer generate CH3(CH2)5COOH. Extract with ether, distill ether to obtain heptanoic acid. p.7 1999 P.I Q.8(b) In an experiment to prepare 1-bromobutane, a mixture of butan-1-ol, potassium bromide and concentrated sulphuric (VI) acid was heated under reflux for 30 minutes. (i) Draw a labelled diagram of the set-up used. (ii) Suggest how to isolate 1-bromobutane from the reaction mixture. (4M) p.8 p.9 Use dropper to remove the aqueous layer / remove aq. component by simple distillation. Shake the organic layer with Na2CO3(aq) / NaHCO3(aq) in a separating funnel until the excess acid has completely been neutralized. Keep the denser organic layer. Add anhydrous Na2SO4 to the organic layer until the liquid becomes clear. Decant liquid to get rid of Na2SO4 particles. Distill liquid and collect vapour at the boiling point of 1-bromobutane. p.10 1995 P.I Q.4(b) (i), (ii) Describe the experimental procedure by which you would recrystallize a sample of impure benzoic acid. How would you test product? the purity of the (4M) p.11 Dissolve in minimum quantity of hot water using boiling tube. Filter the solution while it is hot through fluted filter paper into beaker. Leave for crystals to form. Filter off the crystals and wash with minimum amount of cold water. Dry between filter paper until not damp. Test the purity of product by melting point determination, compare with literature value. p.12 2001 P.I Q.8 p.13 p.14 Other Important Examples (1) 1990 P.1B Q.4(a) Outline a chemical method to separate X, Y and Z from each other. OH NH2 CH3 Br X 1991 P.1B Q.5(a) CH3 X NH2 CH3 Y Z The amine X, C7H9N, may be prepared from Y, C7H7NO2, by the reaction with excess hot granulated tin and conc. HCl. If, in this reaction, some Y remains unreacted, suggest a procedure to isolate pure X from the reaction mixture. Y NO2 p.15 Other Important Examples (2) 1992 P.1B Q.5(b) An incomplete reaction of phenylamine with ethanoic anhydride gave a liquid mixture on cooling. Briefly oultine a procedure, using separating funnel and appropriate reagent(s), to isolate the ethanoylation product. p.16