Exercise on Chapter 3. Structures of Ionic Solids.
advertisement

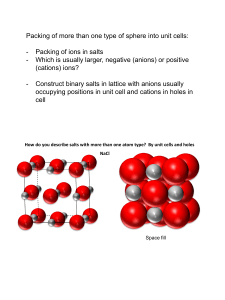
HW# 6 – CHEM 281 Louisiana Tech University, POGIL(Process Oriented Guided Inquiry Learning) Exercise on Chapter 3. Structures of Ionic Solids. Why? Many ionic structures may be described as close-packed arrangements of anions with cations filling the tetrahedral and/or octahedral-shaped holes. A given ionic solid forms a particular structure based on the stoichiometry of the solid and the relative sizes of cations and anions. For a closed-packed structure of spheres, there are two tetrahedral holes and one octahedral hole per large, close-packed sphere. The extent of filling these possible cation sites determines the stoichiometry of the solid. Because the anions can either form hexagonal closepacked or cubic close-packed arrays, there are two possible structures for each stoichiometry. If this is confusing now, it may become more obvious after working through some of the structures below. Tetrahedral holes are smaller than octahedral holes and so will usually accommodate smaller cations. This size distinction between the holes leads to the Radius Ratio Rule for predicting ionic structures. However, the rule of size is not always strictly followed. Larger cations may fill tetrahedral holes for the sake of a particular toichiometry, Resources INORGANIC CHEMISTRY By Peter Atkins, Tina Overton, Jon Rourke, Mark Weller, Fraser Armstrong, 5th Edition 2010. New Concepts The ionic crystal structures: Open Packing: CsCl Structure: A crystal lattice of CsCl (MX) based on AB open packing similar to metals but intertwined A+B+ layers of cations (M+) and A-B- layers of anions (X-) makinga a body-centred cubic (bcc). Pervoskite: A crystal lattice of CaTiO3 (MM’X3) based on AA open packing of Ca2+ ions in a simple cube (sc) unit cell with and 6 O2- ions in the six faces of cube and one Ti4+ in the cubic home of the simple cube. Cubic Close packing (ccp or fcc): Rock Salt Structure: A crystal lattice of NaCl (MX) based on ABC close packing similar to metals but intertwined A+B+C+ layers is cations (M+) and A-B-C- layer of anions (X-) making cubic close packed (ccp) or face centered cube (fcc). Fluorite Structure: A crystal lattice of CaF2 (MX2) based on ABC close packing of Ca2+ ions in a fcc unit cell and 8 F- ions in the tetrahedral holes in the body of the fcc. Sphalrite Structure: A crystal lattice of ZnS (MX) based on ABC close packing of S2- ions in a fcc unit cell and 4 Zn2+ ions in the tetrahedral holes in the body of the fcc. Hexagonal Close Packing (hcp): Wurtzite: A crystal lattice of ZnS (MX) based on ABA close packing of S2- ions in a hcp unit cell and Zn2+ ions in the tetrahedral holes in the body of the hcp. Rutile: A crystal lattice of TiO2 (MX) based on ABA close packing of O2- ions in a hcp unit cell and Ti4+ ions in the octahedral holes in the body of the hcp. Coordination number for both anion and cation: CsCl Structure: Cell M(CN) X(CN) Formula 1 Cesium chloride structure: bcc Cs+(8) Cl-(8) MX 2 Pervoskite structure: sc Ca2+, Ti4+ O2CaTiO3 3 Rock Salt Structure: fcc Na+(6) Cl-(6) NaCl 4 Fluorite Structure: fcc 5 Sphalrite Structure: fcc 6 Wurtzite: hcp 7 Rutile: hcp Pervoskite structure of superconducting material (1-2-3:Y-Ba-Cu) YBa2Cu3O7: Factor affecting the ion coordination number Miller indices of the planes in crystalline lattices: 1. What is radius ratio rule? Radius Ratio Coordination Number Type of Hole for Cation 0.225-0.414 4 Tetrahedral 0.414-0.732 6 Octahedral 0.732-1.000 8 cubic CHEM 281 Name:_______________________________ HW 6. Chapter 3. Ionic Structures Key Questions (relatively simple to answer using the Focus Information) 1. Explain the solid state structures of ionic compounds using the concepts applied for solidstate metallic structures. a) Body-centred Cubic (bcc) CsCl Structure: CaTiO3 Pervoskite Structure b) Cubic Close Packing (ccp or fcc) Rock Salt Structure: Fluorite Structure: Sphalrite Structure: c) Hexagonal Close Packing (hcp) Wurtzite: Rutile: 2. Give coordination number for both anion and cation of the following ionic lattices. a) CsCl Structure: b) Rock Salt Structure: c) Fluorite Structure: d) Sphalrite Structure: e) Wurtzite: f) Rutile: 3. Give the number of ions per unit cell of the pervoskite structure of supercondicting material (1-2-3:Y-Ba-Cu) given below: 4. Explain the factor affecting the ion coordination number in an ionic compound. 5. Why, in the study of an ionic lattice, is the anion packing considered to be the frame into which the cations fit? 6. Draw the Miller indices of the planes in the sodium chloride structures below: 7. What is radius ratio rule? 8. Suggest the probable crystal structure of (a) barium fluoride; (b) potassium bromide; (c) magnesium sulfide. You can use comparisons or obtain ionic radii from data tables. 9. Use a model to draw a partial ionic lattice diagram for the antifluorite structure of lithium oxide 10. In a sodium chloride lattice, the ions usually touch along the edge of the unit cell. If the ionic radii are in r+, and r-, calculate the length of each side of the unit cell. 11. In a cesium chloride lattice, the atoms usually touch along the diagonal from one corner through the center of the cell to the opposite comer. If the ionic radii are r+ and r-, calculate the length of each side of the unit cell. 12. The ions in cesium chloride are arranged in a body-centered cubic unit cell. Calculate the radius of a cesium ion if the density of cesium chloride is 3.97 g-cm-1, and it is assumed that the ions touch through the diagonal of the unit cell.