Chapter 13
advertisement
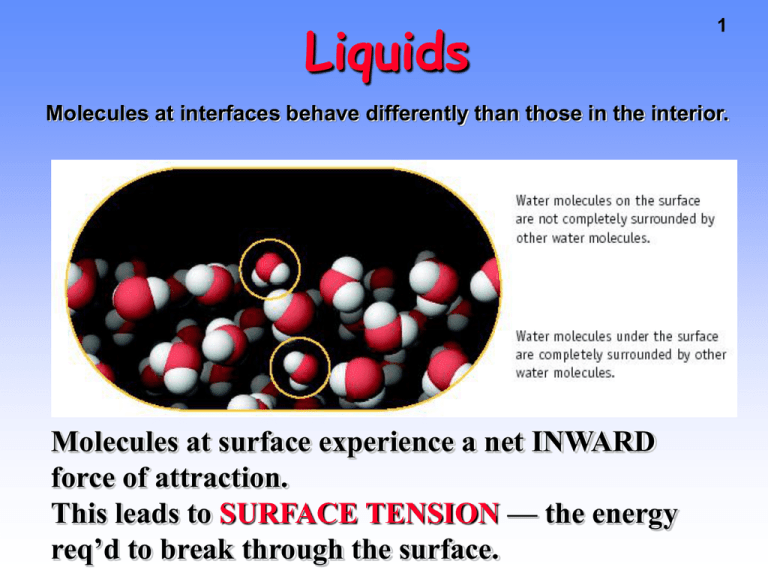
Liquids 1 Molecules at interfaces behave differently than those in the interior. Molecules at surface experience a net INWARD force of attraction. This leads to SURFACE TENSION — the energy req’d to break through the surface. 2 Surface Tension SURFACE TENSION also leads to spherical liquid droplets (shape of minimum surface). Liquids 3 Intermolec. forces also lead to CAPILLARY ACTION and to the existence of a concave meniscus for a water column in a glass tube. concave meniscus H2 O in glass tube ADHESIVE FORCES between water and glass (with polar Si-O bonds) COHESIVE FORCES between water molecules Capillary Action Cohesive forces against the force of gravity Movement of water up a piece of paper depends on H-bonds between H2O and the OH groups of the cellulose in the paper. Problem : Search for applications of capillary action in nature (plants) and in the lab (chromatography) 4 Liquids 5 High surface tension due to cohesive forces stronger than adhesive forces with the glass leads to the existence of a convex meniscus for a column of mercury in a glass tube. convex meniscus Hg in a glass ADHESIVE FORCES between Hg and glass (with polar Si-O bonds) COHESIVE FORCES Non-polar mercury 6 Viscosity VISCOSITY is the tendency or resistance of liquids to flow. Do you expect the viscosity of glycerol to be larger or smaller than the viscosity of ethanol ? Ethanol Glycerol The resistance to flow results from several factors, including intermolecular interactions, molecular shape and size. Metallic and Ionic Solids 7 Sections 13.6-8 Solid-state chemistry is one of the booming areas of science, leading to the development of interesting new materials. Types of Solids 8 Table 13.6 TYPE Composition Ionic NaCl, CaF2, ZnS Metallic Na, Fe Molecular Ice, I2 Network BINDING FORCES Ion-ion Metallic Dipole Ind. dipole Diamond Graphite Amorphous Glass, polyethylene Extended covalent Covalently bonded Networks with no Long-range Regularity. Network Solids Diamond Graphite 9 Network Solids A comparison of diamond (pure carbon) with silicon. 10 Properties of Solids 1. Molecules, atoms or ions locked into a CRYSTAL LATTICE 2. Particles are CLOSE together 3. STRONG IM forces 4. Highly ordered, rigid, incompressible 5. No translations (only vibrations, or rotations on lattice sites) ZnS, zinc sulfide 11 Crystal Lattices • Regular 3-D arrangements of equivalent LATTICE POINTS in space. • Lattice points define UNIT CELLS – smallest repeating internal unit that has the symmetry characteristic of the solid. 12 Cubic Unit Cells There are 7 basic crystal systems, but we are only concerned with CUBIC. All sides equal length All angles are 90 degrees 13 Cubic Unit Cells of Metals Figure 13.24 Simple cubic (SC) 1 atom/unit cell Bodycentered cubic (BCC) 2 atoms/unit cell Facecentered cubic (FCC) 4 atoms/unit cell 14 Units Cells for Metals Figure 13.25 15 Atom Packing in Unit Cells Assume atoms are hard spheres and that crystals are built by PACKING of these spheres as efficiently as possible. 16 Number of Atoms per Unit Cell Unit Cell Type SC BCC FCC Net Number Atoms 1 2 4 17 18 Atom Sharing at Cube Faces and Corners Atom shared in corner --> 1/8 inside each unit cell Atom shared in face --> 1/2 inside each unit cell Simple Ionic Compounds CsCl has a SC lattice of Cs+ ions with Cl- in the center. 1 unit cell has 1 Cl- ion plus (8 corners)(1/8 Cs+ per corner) = 1 net Cs+ ion. 19 Simple Ionic Compounds Salts with formula MX can have SC structure — but not salts with formula MX2 or M2X 20 21 Two Views of CsCl Unit Cell Either arrangement leads to formula of 1 Cs+ and 1 Cl- per unit cell NaCl Construction FCC lattice of Cl- with Na+ in holes Na+ in octahedral holes 22 The Sodium Chloride Lattice Many common salts have FCC arrangements of anions with cations in OCTAHEDRAL HOLES — e.g., salts such as CA = NaCl • FCC lattice of anions ----> 4 A-/unit cell • C+ in octahedral holes ---> 1 C+ at center + [12 edges • 1/4 C+ per edge] = 4 C+ per unit cell 23 24 Comparing NaCl and CsCl • Even though their formulas have one cation and one anion, the lattices of CsCl and NaCl are different. • The different lattices arise from the fact that a Cs+ ion is much larger than a Na+ ion. Phase Diagrams 25 Lines connect all conditions of T and P where EQUILIBRIUM exists between the phases on either side of the line. Phase Equilibria — Water Solid-liquid 26 Gas-Liquid Gas-Solid 27 Phases Diagrams— Important Points for Water Normal boil point T(˚C) P(mmHg) 100 760 Normal freeze point 0 760 Triple point 4.58 0.0098 Solid-Liquid Equilibria In any system, if you increase P the DENSITY will go up. Therefore — as P goes up, equilibrium favors phase with the larger density (or SMALLER volume/gram). Liquid H2O Solid H2O Density 1 g/cm3 0.917 g/cm3 cm3/gram 1 1.09 28 Solid-Liquid Equilibria 29 Raising the pressure at constant T causes water to melt. The NEGATIVE SLOPE of the S/L line is unique to H2O. Almost everything else has positive slope. 30 Solid-Vapor Equilibria At P < 4.58 mmHg and T < 0.0098 ˚C solid H2O can go directly to vapor. This process is called SUBLIMATION This is how a frost-free refrigerator works.