STUDY GUIDE—ELECTRON ENERGIES & CONFIGURATIONS
advertisement

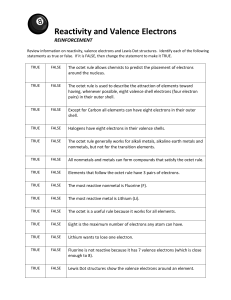
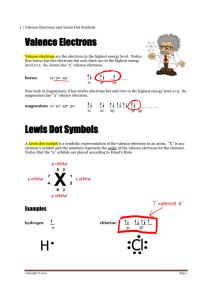
STUDY GUIDE—ELECTRON ENERGIES & CONFIGURATIONS 1. Define wavelength, frequency, amplitude, hertz, valence electrons, octet, and photon. 2. Give the relationship between wavelength, frequency, and energy of a wave. 3. Differentiate between the ground state vs. the excited state of an electron. 4. What did the Bohr model say about electrons? 5. What was incorrect about Bohr’s thoughts on electrons? 6. Why are valence electrons so important? 7. List the 4 sublevel letters in order of increasing energy. 8. Where do electrons move to when they gain energy? 9. What color of visible light has the MOST energy? The LEAST energy? 10. List all of the sublevels beginning with 1s and ending at 7p in order from least to greatest energy. 11. What element family has an octet? 12. Describe 2 practical ways to get electrons excited. 13. Why is an octet chemically unreactive? 14. Give the names of the families with s valence electrons. 15. Give the names of the families with p valence electrons. 16. Give the names of the family with d valence electrons. 17. Give the names of the families with f valence electrons. 18. What rows have s and p sublevels being filled? 19. What rows have the d sublevel being filled? 20. What rows have the f sublevel being filled? 21. If an atom has 5 valence electrons, how many MORE does it need to complete the octet rule? 22. What do Lewis dot structures show? 23. Why do atoms form chemical bonds? 24. Write the entire configuration AND Dot diagrams for: Se, Rh, Zr, Pt, Fm, Bh, Ra, Sb 25. Write the ABBREVIATED configuration AND Dot diagrams for: Te, Mn, Pb, Lr, Ac, Rn, Po, Ga, Cs, Zn, I