Department of Physiology Safety & Health
advertisement

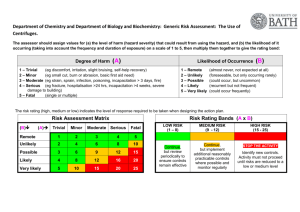
Department of Physiology Procedure No: PHY/SOP/EQ01 Revision No: 001 Title: Effective Date : 14/6/2010 Centrifuge Safety (Adopted from OSHE/SOP/BS/06) Page: Page 1 of 6 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 Prepared/Review By Approved By Next Review Date Oh Chu Yun Dr. Leung Pui Lam Bernard 14/6/2013 * Review Date = Future date for the next revision (every 3yrs) 1.0 OBJECTIVE The objective of this document is to provide guidance on how centrifuges are to be used, cared for and maintained in a safe manner. Centrifuges must be properly used and maintained because the rotors are subjected to powerful mechanical stress that can result in rotor failure. In addition, improper loading and balancing of rotors can cause the rotors to break loose while spinning leading to damage. 2.0 SCOPE This procedure is applicable to all laboratories using centrifuges in NUS, Physiology. 3.0 RESPONSIBILITIES 3.1 Principal Investigator (PI) The PI is to ensure that: a. Operating instructions, users’ manuals, repair and maintenance histories are available for centrifuges under his/her charge. b. Rotor logs are kept for high speed and ultra-centrifuges. See Appendix 1 for the recommended rotor log format. c. All users are trained and competent, and training records are kept. d. Users observe all instructions for safe and responsible operation as detailed in this procedure. e. Ensure that the person who is responsible for each centrifuge carries out all the described safety & health tasks (including regular maintenance, servicing and record keeping). f. Appropriate warning signs are provided in rooms where potentially hazardous biological, radioactive materials, toxic or other hazardous chemicals are being centrifuged. g. All incidents or lapses are reported to the Department Safety & health Officer. Department of Physiology Procedure No: PHY/SOP/EQ01 Revision No: 001 Title: Effective Date : 14/6/2010 Centrifuge Safety (Adopted from OSHE/SOP/BS/06) Page: Page 2 of 6 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 3.2 Centrifuge operator The operator is responsible to: a. Attend training identified by his/her PI. b. Know and follow operating instructions for rotors and centrifuges used. c. Safely handle, operate and clean all centrifuges and rotors during and after use. d. Fill out the log each time the equipment is used (applicable for high speed and ultracentrifuges only). e. Clean spills or breakage within centrifuge, noting and reporting concerns regarding possible damage, misuse, and/or failure of other users to comply with this procedure. f. Report damage to centrifuge or rotor to the PI so that action in terms of repair or decommissioning may be performed. 3.3 Designated Person The Head of Department shall appoint a Designated Person to take charge of the centrifuges that form part of the common facilities. 4.0 DEFINITION 4.1 Classes of Centrifuges Centrifuges are generally divided into three classes: a. Low Speed (up to 15,000 rpm) b. High Speed (15,000 rpm to 25,000 rpm) c. Ultracentrifuge (25,000 rpm or higher) 5.0 PROCEDURES 5.1 Documentation a. Prior to operation of any centrifuge, the user shall review the Manufacturer’s Manual to understand the proper operating procedures for the specific unit being operated. b. A copy of the Manufacturer’s Manual and Safety & Health Manual must be kept in a designated location in the laboratory for easy access. c. A copy of Appendix 2 – Centrifuge Safety should be posted next to a centrifuge for guidance to users. 5.2 Safe Operation a. Lids shall be closed at all times during operation. b. Rotors must be used only with the correct centrifuge. c. Observe maximum speed and sample density ratings designated by the manufacturer for each rotor, and speed reductions required for running high-density solutions, plastic adapters, or stainless steel tubes. Department of Physiology Procedure No: PHY/SOP/EQ01 Revision No: 001 Title: Effective Date : 14/6/2010 Centrifuge Safety (Adopted from OSHE/SOP/BS/06) Page: Page 3 of 6 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 d. The user shall not leave the centrifuge until full operating speed is attained and machine appears to be running safety without vibration. e. If vibration occurs the centrifuge should be stopped immediately and load balances checked. Swing-out buckets should be checked for clearance and support. f. Sample loads must be balanced and swinging bucket rotors must not be run with missing buckets. (½ g at 1 G is approximately equivalent to 250 kg @ 500,000 G’s!) g. Plastic centrifuge tubes should be discarded after one cycle of ultracentrifugation. The failure rate for used tubes is a hazard which justifies the use of new tubes for each high G centrifugation. h. Nitrocellulose tubes should be used only when transparent and flexible (fresh). They must never be heated because of explosive possibility. i. Store all fixed angle vertical tube and near-vertical tube rotors upside down, with the lids or plugs removed. Swinging bucket rotors should be stored with the bucket caps removed. j. If vibration occurs, stop the run immediately; wait until the rotor stops, and check the load balances. k. In the event of a power failure, do not try to open the lid to retrieve samples for at least half an hour. Follow the instructions in the manual for recovery of the samples. 5.3 Working with Hazardous Materials a. Rooms where potentially hazardous biological, radioactive materials, toxic or other hazardous chemicals are centrifuged must be identified by the appropriate warning signs. b. With biohazard materials, rotors must have aerosol containment (“O-rings” and safety cups) or be used in the biological safety cabinet. Rotors must be loaded and unloaded in a biological safety cabinet. With radioactive material, keep centrifuge within an appropriate shield. 5.4 Personal Protective Equipment (PPE) a. Appropriate personal protective equipment like respirators, face shield, goggles and gloves shall be used when required. b. Risk assessment must be conducted to decide on the type of PPE required for the work. 5.5 Emergency Procedures – Centrifuge Spill a. Turn off centrifuge, notify others in laboratory and evacuate if necessary. b. Post temporary hazard warning sign. c. Notify PI, responsible person or departmental safety representative. d. Refer to the relevant Spills procedures (CBL/SOP/02) and report the incident (CBL/SOP/GS/01). Department of Physiology Procedure No: PHY/SOP/EQ01 Revision No: 001 Title: Effective Date : 14/6/2010 Centrifuge Safety (Adopted from OSHE/SOP/BS/06) Page: Page 4 of 6 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 5.6 Maintenance a. Refer to user’s manual for detailed maintenance and care of the centrifuge. b. Rotors and cups are to be cleaned after each use with non-corrosive (non-alkaline for anodized rotors) cleaning solutions and stored inverted when required by the manufacturer. Mild detergent is recommended. c. After proper clean-up, rinse the rotor with de-ionized or distilled water. d. Sharp plastic bristle tube brushes are not to be used for cleaning cavities. Remove all trace of detergents before air drying. e. Keep rotors clean and dry. If spills occur, make sure rotor has been cleaned / decontaminated. If salts or corrosive materials were used, ensure they have been removed from the rotor. f. Avoid mechanical scratches. The smallest, scarcely visible scratch allows etching to enlarge the fracture, which is subject to enormous rupturing forces at high G’s – a vicious cycle leading to rotor explosion. g. Rotors shall be retired after the manufacturers’ recommended revolutions or years of service, whichever comes first, except where an annual stress test (magnaflux or other professionally recognized analysis) proves an absence of structural flaws. h. If a centrifuge is experiencing problems or maintenance is required, the unit must be immediately taken out of service, disconnected from the power source and clearly marked DO NOT USE until serviced. This notice will include the name of the person, the date, the reason and the signature of the PI or the Responsible Person. 6.0 RECORDS Rotor Log is to be kept for one year. 7.0 REFERENCES a. OSHE/BS/GL/03 – Biological Spill procedure b. OSHE/U/GL/01 – Selection of PPE c. OSHE/SOP/U/02 – Chemical and Biological Accidents / Incidents Reporting and Investigation 8.0 APPENDICES Appendix 1: Recommended Rotor Log Format Appendix 2: Centrifuge Safety Poster Department of Physiology Procedure No: PHY/SOP/EQ01 Revision No: 001 Title: Effective Date : 14/6/2010 Centrifuge Safety (Adopted from OSHE/SOP/BS/06) Page: Page 5 of 6 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 Department of Physiology Procedure No: PHY/SOP/EQ01 Revision No: 001 Title: Effective Date : 14/6/2010 Centrifuge Safety (Adopted from OSHE/SOP/BS/06) Page: Page 6 of 6 Safety & Health Laboratory (Location): Appendix 2 Cytokine biology Lab, MD9