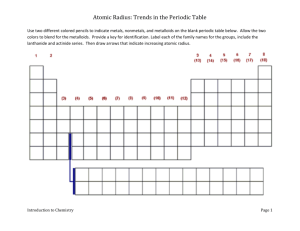
Lab - Periodic Trends

Objective: To predict the atomic radius of elements by graphing the atomic radius vs. atomic number.
1.
become familiar with the organization of the periodic table
2.
study a periodic property
3.
make predictions based on periodic properties
Procedure:
1.
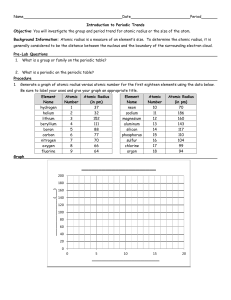
Using the data in Table 1, plot the atomic radius of each element on the y-axis against its atomic number on the x-axis. (For full credit, graph must have straight lines. Use a ruler!) a.
Choose the scales so that all the data will fit on the sheet. b.
Connect each two consecutive points with a straight line. c.
Label each peak with the symbol of the element.
2.
For Mg and Fe; skip their plot point when plotting the points. When connecting the points, connect Na to Al and Mn to Co with a straight line.
Si
P
S
Cl
Ne
Na
Mg
Al
Ar
H
He
Li
Be
B
C
N
O
F
Table 1 Atomic Radii of Elements in nm
Element Atomic
Number
1
2
14
15
16
17
10
11
12
13
7
8
9
3
4
5
6
18
Atomic Radius
0.037
0.050
0.152
0.111
0.088
0.077
0.070
0.066
0.064
0.070
0.188 unknown
0.143
0.117
0.110
0.104
0.099
0.094
Element
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Atomic
Number
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
Atomic Radius
0.231
0.197
0.160
0.146
0.131
0.125
0.129 unknown
0.125
0.124
0.128
0.133
0.122
0.122
0.121
0.117
0.114
0.109
Questions (Complete sentences):
1.
Describe any regularities that are present in the graph of atomic radius versus atomic number.
2.
Which elements occupy the “peaks” in the cycles?
3.
Would you expect the elements at the “peaks” which have large atomic radii to have large or small ionization energy (the energy required to lose valence electrons)? Explain your answer
4.
Which elements occupy the “valleys” in the cycles?
5.
Would you expect the elements at the “valleys” which have small atomic radii to have large or small ionization energy? Explain your answer
6.
How many elements separate each peak? How does this correlate to the periods on the periodic table?
7.
In Table 1 the atomic radii of Mg and Fe blank intentionally. The part of the cycle from Li (3) to B
(5) is repeated in the part of the cycle from Na (11) to Al (13). One would then expect that the radius of Mg would have a value that would maintain the pattern observed between Li and B.
On that basis, predict the radius of the Mg atom from the graph
8.
As part of the last cycle on the graph, which starts at K (19), we have the first series of transition metals. By assuming that the Fe (26) atom would maintain the pattern set by atoms near it in atomic number, predict the radius of the Fe atom from the graph.
9.
Using your periodic table and the pattern on the graph would you predict Rb to have a smaller or larger atomic radius than K? Explain why.