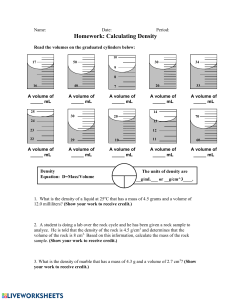
Identifying Substances
advertisement
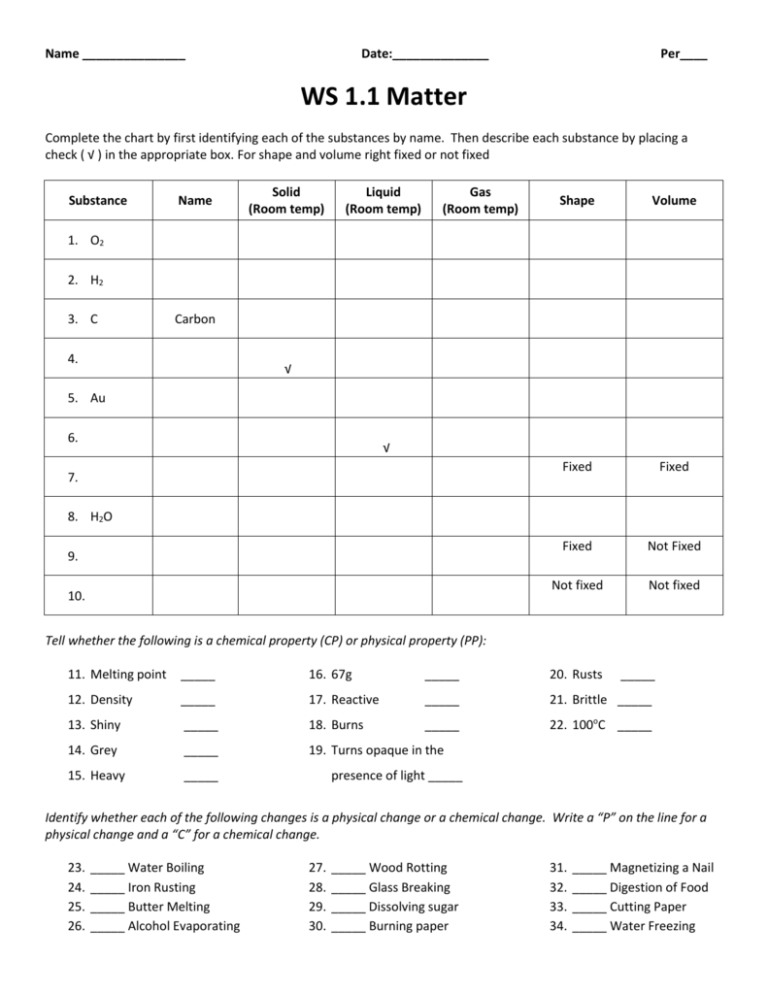
Name _______________ Date:______________ Per____ WS 1.1 Matter Complete the chart by first identifying each of the substances by name. Then describe each substance by placing a check ( √ ) in the appropriate box. For shape and volume right fixed or not fixed Substance Name Solid (Room temp) Liquid (Room temp) Gas (Room temp) Shape Volume Fixed Fixed Fixed Not Fixed Not fixed Not fixed 1. O2 2. H2 3. C Carbon 4. √ 5. Au 6. √ 7. 8. H2O 9. 10. Tell whether the following is a chemical property (CP) or physical property (PP): 11. Melting point _____ 16. 67g _____ 20. Rusts _____ 12. Density _____ 17. Reactive _____ 21. Brittle _____ 13. Shiny _____ 18. Burns _____ 22. 100oC _____ 14. Grey _____ 19. Turns opaque in the 15. Heavy _____ presence of light _____ Identify whether each of the following changes is a physical change or a chemical change. Write a “P” on the line for a physical change and a “C” for a chemical change. 23. 24. 25. 26. _____ Water Boiling _____ Iron Rusting _____ Butter Melting _____ Alcohol Evaporating 27. 28. 29. 30. _____ Wood Rotting _____ Glass Breaking _____ Dissolving sugar _____ Burning paper 31. 32. 33. 34. _____ Magnetizing a Nail _____ Digestion of Food _____ Cutting Paper _____ Water Freezing Complete the following density problems. You must show all work to receive credit. Remember to include your units. (NOTE: 1ml = 1cc= 1cm3) 35. A block of aluminum has a mass of 36.40 g and a volume of 13.5 cm3. What is the block’s density? 36. A cup of salt water has a volume of 12.00ml and a mass of 13.16g. What is the cups density? 37. The density of CCl4 (carbon tetrafluoride) is 1.58 g/mL. What is the mass of 95.7 mL of CCl4? 38. What is the volume of 227 g of olive oil if its density is 0.92 g/mL? 39. Rock is often an irregular shape and so its volume can’t be determined by simple means. The water displacement method is often used to determine the volume of irregularly shaped objects. A graduated cylinder has an initial volume of 50.0ml of water. After a rock is added the volume of water in the graduated cylinder reads 65.5ml. The mass of the rock is found to be 20g. what is the rocks density? Initial volume of water Final volume of water Volume of rock Mass of rock 40. Equal amounts of three liquids – carbon tetrachloride (D = 1.58 g/cm3), mercury (D = 13.546 g/cm3), and water (D = 1.00 g/cm3) – are mixed together. After mixing, they separate to form three distinct layers. Draw and label a sketch showing the order of the layers in the test tube. Answers: 246.74 1.097 1.29 151.206 1.097