Density Worksheet.
advertisement

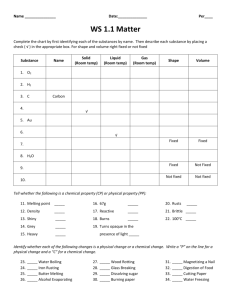
In the SI system, volume can be expressed in two ways, one is in liters and the other as a unit of distance “cubed” such as cm3. When using distance the “cube” is because you must multiply a distance times a distance times a distance. It is very important that all three distances are in the same unit such as cm or m or mm. For a cube the formula is length X width X height. Once you have the volume and the mass, it is easy to calculate the density of an object, that is, the amount of stuff in a certain space. To find the density, the formula is mass/volume. Work the following problems for practice; you’ll need it on an upcoming lab and chapter test. 1. What is the volume of a box measuring 1m X 5m X 6m? (Remember units) 2. What is the volume of a box measuring 2cm X 7cm X 3cm? 3. What is the volume of a cube measuring 5cm on each side? You must have the correct UNITS in order to get credit! 4. What is the volume of a box measuring 3cmX6cmX4cm? 5. What is the volume of a box measuring 8mm X 10cm X 5cm? (Be careful – units!) For the next problems you’ll need to figure density. Remember that density is mass/volume. 6. An iron cube measures 10cm X 10cm X 10cm. What is its volume? 7. If the same iron cube weighs 7.9g, what is its density in g/cm3? 8. What is the density of a cube of water measuring 2cm X 4cm X 1cm, with a mass of 8g? 9. What is the density of a block of wood measuring? 9cm X 2cm X 6cm with a mass of 5.4g 10. A rock has a mass of 210 grams and occupies a volume of 70 cm 3. What is its density? 11. An unknown liquid occupies a volume of 5 ml and has a mass of 40 grams. Find its density. 12. A rock occupies a volume of 20 cm3 and has a mass of 54 grams. Find the density of this rock. 13. A graduated cylinder has 20 ml (cm3) of water placed in it. An irregularly shaped rock is then dropped in the graduated cylinder and the volume of the rock and water in the cylinder now reads 30 ml (cm3). The mass of the rock dropped into the graduated cylinder is 23 grams. a Find the volume of the rock dropped into the graduated cylinder. b Find the density of the rock dropped into the graduated cylinder. Use the table below to answer the following questions: Substance Wood Corn oil Water Density at 20 ◦C 0.70 g/cm3 0.92 g/cm3 1.00 g/cm3 Substance Rubber Corn Syrup Copper Density at 20 ◦C 1.34 g/cm3 1.38 g/cm3 8.80 g/cm3 14. An object with a mass of 24g and a volume of 32mL is most likely what substance is it? 15. What is the only object in the table that would sink in corn syrup? ____________ 16. What is the mass of 100 mL of corn oil? 17. What is the volume of 35g of copper? Using Graduated Cylinders Directions: What does each of the graduated cylinders shown below read? Directions: What lengths are marked on the centimeter ruler shown? cm mm A B C D E Directions: Measure each of the following lines with a centimeter ruler. Record your answers on the lines at right. F. ____________ G. ______________________ H. ____________________________________ I. ___________________________ J. ______________________________________________ K. ___ L. _____________________________________________ Volume of a Rectangular Solid The volume of a rectangular solid is given by the formula V = LWH where V = VOLUME, L = length, W = width, and H = height. Calculate the volume of each of the following solids. Using Triple Beam Balances What mass is shown on each of the following balances? Write your answer in the left margin.