File
advertisement

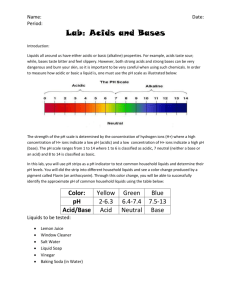
Name___________________________Period_____ Acids and Bases pH Lab Answer Sheet Complete this chart for the Results (#2). Complete the final question below this chart (#10). Answer all other lab questions (#1 and #3-9) on the back of this chart. Substance pH indicator paper number Phenol Red (only for vinegar and lemon juice in mini-cups) Acid, Base, or Neutral? Distilled Water Lemon soda Ammonia Vinegar Milk Dish soap Lemon juice Cola Soda Rubbing Alcohol 10) Look at the following formulas for some of the substances. How do their formulas reveal whether they are acids or bases and did the formulas accurately tell you if they were acids or bases? Explain. 1. Distilled Water H2O 2. Lemon Soda (solution – homogeneous mixture – not a pure substance - no chemical formula) 3. Ammonia NH4OH 4. Vinegar C2H4O2 5. Milk (colloid- heterogeneous mixture – not a pure substance - no formula) 6. Dish soap (solution – homogeneous mixture – not a pure substance – no formula) 7. Lemon Juice Citric Acid has a formula – C6H8O7 8. Cola Soda (solution – homogeneous mixture – not a pure substance – no formula) 9. Rubbing Alcohol C3H8O When finished: Read and answer questions in Ch 7 Lessons 2 and 3. There could be questions on FCAT related to this! ACIDS AND BASES LAB Purpose: To test common household substances with indicators in order to classify substances as acid, base, or neutral. 1) Hypothesis: I predict the following substances will be… acids_________________________________________________________________________ bases_________________________________________________________________________ neutral________________________________________________________________________ Materials: pH Paper Goggles Test Substances Testing Trays Toothpicks Procedure: For all the test substances complete the following and put your answers in the data table below. a. Place a few drops of each test substance in the designated well of testing tray. Place the substances in the order given in the data table. b. Carefully dip a strip of pH paper into the substance and hold for just a second. Then take it out. c. Record the color results in the data table. d. Repeat for each substance. e. Next, your teacher will add a few drops of phenol red indicator to each substance in the cups. Mix it up with a toothpick and record the color in the data table. f. When done bring your testing tray to your teacher. All other materials go in the trash can. KEEP YOUR GOGGLES ON DURING CLEAN UP! 2) RESULTS: DATA TABLE (on separate paper) ANALYSIS Questions: 3) What is the pH range for acids, bases, and neutral substances? Acid pH____________________ Neutral pH___________________ Base pH____________________ 4) How can you tell and acid from a base when looking at a chemical formula? CONCLUSION – Answer all questions below 5) Was your hypothesis correct? If not, explain why? 6) What did you learn about indicators and how they change with acids and bases? 7) How is the pH scale used to classify acids, bases, and neutral substances? 8) Was there a control used in this test? So is this an investigation or an experiment? Explain. 9) What were the independent and dependent variables in this test? List the variables and define why they are those variables. 10) The question is on your answer sheet.