Properties of Water Notes
advertisement

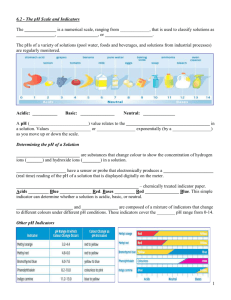
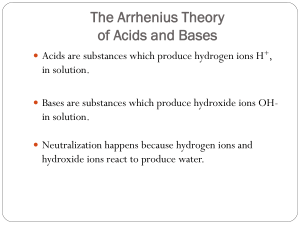
Properties of Water Notes Chapter 2-2 (pg. 40-42) A water molecule is made up of three atoms: 1 ___________________________ and 2 __________________. This compound makes it a very unique molecule. It is unique because it is ____________________. Polarity means that it has an uneven distribution of electrons between the oxygen and the hydrogens. Water is also known as The Universal ________________________. It has 2 major properties: _______________ and ____________________. 1. Cohesion is an attraction between _____________________ substances made possible by _____________________ bonds. (This is why water is attracted to itself – i.e water drops get bigger and bigger). The property of cohesion produces a surface film on water called __________________ _____________________ which allows insects to walk on water and leaves to float on water. Surface tension is a measure of the __________________ of the water’s surface. 2. Adhesion is an attraction between _____________________substances also made possible by ___________________ bonds. (This is why paper towels can soak up water and water forms a meniscus inside of a graduated cylinder). Adhesion and cohesion together cause a process called ___________________ _______________. This action allows water to pull other water molecules along in a stack as it “climbs” up a structure. Example: the drawing of water out of the roots of a plant and up into its stems and leaves.. Water has a very high specific heat which is important in living organisms for maintaining ___________________________ in regard to internal body temperature. Water also has a high heat of ________________________ in which water is converted from a liquid to a gas. As water evaporates it removes __________ with it. Example: ____________________________________________________________ Water is also _______________ _______________ as a solid than a liquid. This is why ice floats. Water molecules ___________________ when they become solid. A ________________________________ is any substance that dissolves other substances. A _________________________ is a mixture of 2 or more substances that are dissolved by the solvent. Example: _______________________________________ A _______________________ is a material composed of 2 or more elements or compounds that are physically mixed together but does not chemically combine. An example would be mixing salt and pepper. Substances that dissolve easily in water are said to be water soluble or _______________________________ (which means ___________ ______________). Substances that do not dissolve easily in water such as fats, oils and waxes are said to be insoluble or _____________________________ (which means ______________ _____________________). Acids & Bases: One water molecule in 550 million will naturally dissociate into a hydrogen ion (H+) and a Hydroxide ion (OH-). Chemists devised a measurement system called the pH scale to indicate the concentration of H+ ions in solutions. This pH scale ranges from 0 to 14. Pure water has an equal number of hydrogen ions and hydroxide ions making its pH value _____. ____________ contain higher concentrations of H+ ions than pure water and have pH values that range from _________ to _________. _____________ contain lower concentrations of H+ ions than pure water and have pH values that range from ________ to _________. __________________ are weak acids or bases that can react with strong acids or bases to prevent sharp and sudden changes in pH which is important to maintaining ___________________________.