Matter Test Review
advertisement
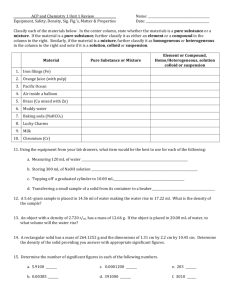
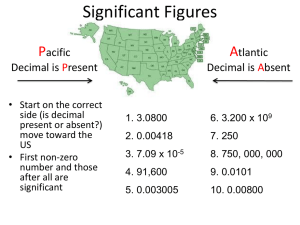
Matter Test Review Name______________________________________________________________Period____________ 1. In your own words, what are the sig fig rules concerning zeros (and give examples in your answers). 2. How many sig figs are in the following numbers? a. 605 ________ c. 0.000056 ________ b. 1010 ________ 3. d. 5.7805 x 10-4_________ Round the following to 3 sig figs. a. 605.24 ___________ c. 0.005699 ___________ b. 1010 ___________ e. 0.0080 _________ f. 907,500,000 _________ e. 579,99999 ___________ d. 5.7805 x 10-4 ___________ f. 6.722 ___________ 4. Add. Subtract. Multiply. Divide. Express your answer with the correct number of sig figs. a. 56.5 + 36.4555 = c. 678.342 / 35.44 = b. 1.5656 x 1 = d. 56 - 0.0423 = 5. Draw a picture of what the atoms look like in each type of matter: Monatomic Element Diatomic Element Compound A mixture of elements Solution A mixture of an element and compound Suspension A mixture of compounds 6. What is the difference between an intensive and extensive property? What type of property can these terms be used to describe? 7. Write 3 examples of intensive properties: Write 3 examples of an extensive properties: 8. Classify each as a chemical property, physical property, chemical change, or physical change. a. b. c. d. Mixing peas and carrots. Burning propane. Dissolving sugar in water. Ability to react with acid. e. Ice subliming to steam. f. Tensile strength. g. Smashing a pumpkin. h. Digesting an apple. i. Reactivity. j. Shaking salad dressing. k. Ductility. l. Mixing baking soda and vinegar. 9. Classify as a substance (SUB) or mixture (MIX). Finally, classify as an element (E), compound (C), solution (SOL), or suspension (PEN). You may have to look up the chemical formulas for some of these. a. b. c. d. e. Iron Steel Calcium carbonate Dirt Egg Drop Soup f. Windex g. Kool-Aid h. Blood i. Diamond j. Helium k. Raisin Bran l. Water m. Lemonade n. NaOH o. Carbon dioxide 10. Does liquid take up a different amount of space when it is put into a different container? Why or why not? 11. Circle the physical changes: Condensation Deposition Combustion Acidification Evaporation Dissolving Melting Tearing Sublimation Freezing Reaction Breaking down 12. A shrinking puddle is an example of ________________________________. 13. Frost on the grass on a cold morning is an example of ____________________________. 14. A disappearing ice sculpture is an example of ______________________________. 15. What do questions 12-14 have in common? 16. How are the processes in questions 12-14 different?