EOG Matter Vocab - New.Schoolnotes.com
advertisement

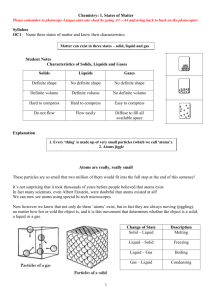
Vocabulary #1 matter Matter Matter is everything that has mass and takes up space. The substance a physical object is made up of. Made up of small particles called ATOMS. Matter and Atoms have mass and take up space. (MEA) Physical property of matter Characteristics that are observable and measurable EX. Six shape temp, color, texture. The things that can be measured without changing the chemical property Element Pure. Can’t be broken down. Make up ALL the things on Earth. All Atoms of the same Elements have the same property. EX. All matter made of iron has the same property. (MEA) Atom Smallest part of an element. All elements are made of atoms. Matter is made of elements. Elements are made of atoms. (MEA). Particles Small piece or part Thermal energy Heat energy Change in phase Changing a material or mixture from one state of matter to another. Matter changes from one state to another. Atoms move faster and spread out more when heated Phase Stages: 4 forms: Solid, liquid, gas, plasma (In which matter can exist) Boiling Point Temperature water boils Melting Point Temperature at which solids melt Pure Substances Matter that CAN NOT be SEPARATED into any other matter by physical process. Solubility The amount of solute that can dissolve in a solvent. The TEMPERATURE of the solvent determines the solubility of that substance/item. The ability of one substance to dissolve into another substance Solute Solid part of a solution Solvent Liquid part of a solution. WATER is the most common solvent Density The amount of Matter in a given volume. The more MATTER you put in a defined space (volume), the more DENSER it gets. Mass The more STUFF (matter) you put in a SPACE (volume), the more the MASS will increase, so the more the DENSITY. Physical change Volume A change takes place, but NOT in the molecular composition of the substance. (not a chemical change). The change may be: tearing one piece of paper into two pieces. The amount of SPACE something takes up Molecules movement when heated: Heated/solid Molecules shake in a tight space./not moving apart-they just VIBRATE Heated/liquid Molecules move a little from each other Heated/gas Molecules move very fast and far away from each other. The hotter ----the more the energy—the more the movement---the more the EXPANSION. SOLIDS/ atoms are not compressible Very small movements. Definite volume and shape. Atoms are packed together. Atoms vibrate because of thermal energy. Solid to a liquid You must add heat. Definite volume but can change shape. The particles touch BUT the particles can move around. They take the shape of the container because the liquid molecules can move. Liquid to gas When heat is added it has no definite shape or volume. Gasses can keep on spreading and spreading out indefinitely. NOTE: Density, melting point, boiling point, and solubility DO NOT CHANGE based on the amount of matter present. EX: Ice will always melt at 32 degrees F. Weather there is a lot of ice or a little bit of ice. NOTE: Volume, Mass, and Weight CHANGES based on how much stuff (MATTER) you have present.