The Stuff that things are made of
advertisement

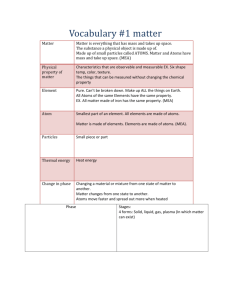
What do all of these things have in common? Matter is anything that has MASS and takes up SPACE Scientists usually measure mass like weight However: Weight differs on different planets, but mass stays the same- why? Matter can be broken down into groups called elements. Elements are PURE substances. They are substances that cannot be broken down into anything else by chemical means. In other words- the simplest ingredients possible on earth to make other things Elements have specific properties. (which means elements act certain ways- specific mass, specific reactions Examples: Gold, Aluminum, Copper, Oxygen, Helium, Potassium, Tin, Silver, Neon The Smallest Unit of an element of matter that can still retain its properties as that element In other words- IT is the smallest piece possible of an element NUCLEUS PROTON (+) NEUTRON (0) ELECTRON () PROTONS (+) NUCLEUS NEUTRONS (0) ELECTRONS (-) # of PROTONS=ELECTRONS Change the protons change the atom Change the electrons change the charge Molecules and Compounds • Two or more atoms bonded together • They can be the same, or they can be different • Remember Bill Nye said Atoms are like the letters, while molecules are like the words. We represent ATOMS as either a single capital letter or a Capital letter and a lowercase letter. ◦ Oxygen = O --- Lithium = Li Molecules are when we put these letters together. EXAMPLE: + = + Hydroge n molecul e = Water molecul e Two or more atoms bonded together. But the atoms that are bonded MUST be different kinds or atoms. All COMPOUNDS are molecules, but not all MOLECULES are compounds. ICE CREAM ANALOGY BASKIN ROBINS 31 flavors Flavors- elements Smallest serving= 1 scoop its like 1 atom 2 + scoops = molecule 2 different flavored scoops a compound Make a T chart like below and put the correct symbols in the correct column. Element Molecule Compound Li CO2 O2 H2 Br OHH2O He C6H12O6 1 person out straight= element 1 person in ball = atom 2 or more people= molecule 2 or more people but there must be a boy and a girl= compound Phases of Matter Remember Matter is everything! /anything that has mass ( a specific amount of atoms) and takes up space We usually find matter in three forms on earth (although scientists now believe there are 5 forms) The phases of matter are the different forms you can find matter in on earth. The state of matter that has definite shape and definite volume. (They are fixed, they don’t change). The atoms and particles in a solid are very close together. Here the attractions are the strongest The state of matter where there is definite volume but not a definite shape. The particles in liquid slide past each other so the attractions aren’t as strong. LIQUIDS TAKE THE SHAPE OF THEIR CONTAINERS The state of matter where the matter has no definite shape, or definite volume. The particles move so quickly they can break away from each other there is less attraction. Gases may also take the shape of their container, but the particles also can spread out Phase of Matter Solid Liquid Gas Definite Shape Definite Volume Takes Shape of Container Fastest moving molecules Phase of Matter Solid Liquid Gas Definite Shape Definite Volume Takes Shape of Container Fastest moving molecules ↯ ↯ ↯ ↯ ↯ ↯ GUESS WHAT?!!!! IT”S FINALLY LAB DAY!!!!!!!!! MATTER is what???? ◦ TAKES UP SPACE ◦ HAS MASS A measure of the amount of matter or stuff inside and an object We usually measure mass as weight. It changes with gravity The amount of SPACE that something occupies. We measure volume by using multiplication ◦ Length of an object X Height of object x Width of the object = L x W x H ◦ If we cannot use a ruler to get a very accurate reading we use the water displacement method to tell us the volume. Measuring continued A ratio of how much mass is in an object compared to how much space it takes up Ratios are represented as fractions M__ V