Vocabulary
advertisement

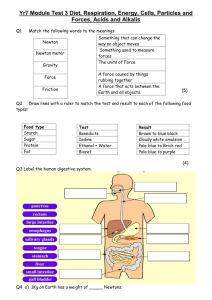
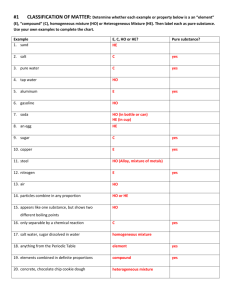
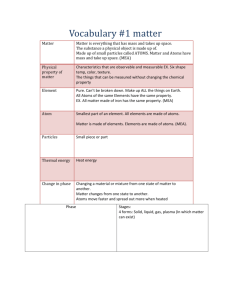
NAME: __________________________________ FOSS Science Mixtures and Solutions Vocabulary Investigation 1 mixture – two or more materials together matter – something that occupies space liquid – state of matter that has mass, takes up space, has a definite volume, but has no definite shape solid – state of matter that takes up space, has a definite shape and volume gas – state of matter that is colorless, takes up space, but does not have a definite volume or shape property – characteristics of an object solution – special mixture formed when a material dissolves in water dissolving – process by which a solid material seems to disappear into a liquid diatomaceous earth – skeletal remains of aquatic organisms, looks like powder mass – a measure of the amount of matter in an object evaporation – process of a liquid drying up; it turns into a gas and goes in to the air crystal – solid form of a material that can be identified by its properties, such as shape, color, and pattern soluble – defines a material that dissolves Investigation 2 solvent – the liquid part of a solution solute – the matter that dissolves in a solution saturated solution – when a solute dissolves in a solvent until no more will dissolve particle – tiny piece of matter Investigation 3 atom – the smallest particle of an element, all matter on earth is made of atoms molecule – particles made of two or more atoms that are held together with bonds compound – substance made of two or more different kinds of atoms chemical reaction – process in which two or more substances combine to make one or more new substances that have different properties than the original ones reactant – the starting substance(s) in a chemical reaction product – substance(s) produced in a chemical reaction chemical formula – code that tells how many and what kind of atoms are in a substance chemical equation – model of a chemical reaction showing reactants and products Investigation 4 element – substance that cannot be broken down by simple chemical land physical processes chemical property – characteristic that describes how a substance is changed when it reacts with another substance periodic table of elements – a way to organize the elements based on atomic number metal – elements that may be shiny, stretch and bend, but don’t break, and conduct heat and electricity well alloy – a mixture of two or more metals