Dalton`s Law Ideal Gas Law Graham`s Law
advertisement
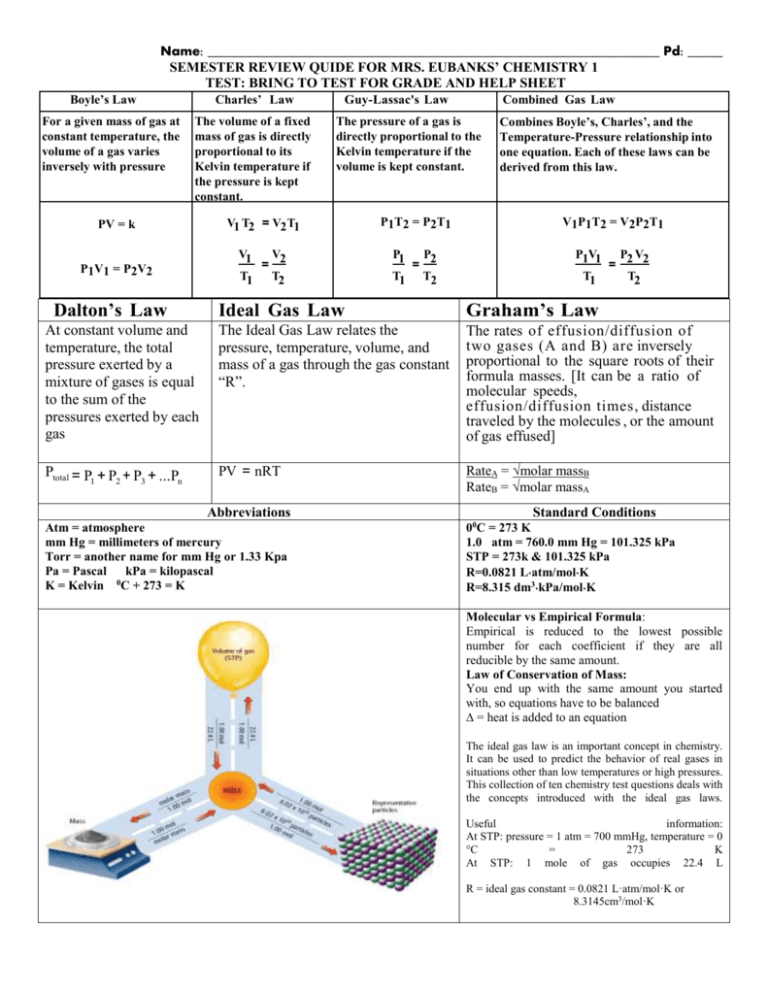
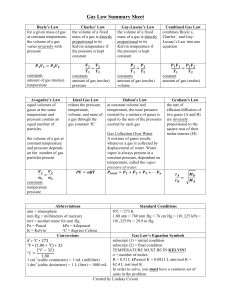
Name: _________________________________________________________________ Pd: _____ SEMESTER REVIEW QUIDE FOR MRS. EUBANKS’ CHEMISTRY 1 TEST: BRING TO TEST FOR GRADE AND HELP SHEET Boyle’s Law For a given mass of gas at constant temperature, the volume of a gas varies inversely with pressure Charles’ Law The volume of a fixed mass of gas is directly proportional to its Kelvin temperature if the pressure is kept constant. Guy-Lassac's Law Combined Gas Law The pressure of a gas is directly proportional to the Kelvin temperature if the volume is kept constant. Combines Boyle’s, Charles’, and the Temperature-Pressure relationship into one equation. Each of these laws can be derived from this law. PV = k V1 T2 = V2 T1 P 1 T 2 = P 2 T1 V 1 P 1 T 2 = V2 P 2 T 1 P1V1 = P2V2 V1 V2 = T1 T2 P1 P2 = T1 T2 P1V1 P2 V2 = T1 T2 Dalton’s Law Ideal Gas Law Graham’s Law At constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the pressures exerted by each gas The Ideal Gas Law relates the pressure, temperature, volume, and mass of a gas through the gas constant “R”. The rates of effusion/diffusion of two gases (A and B) are inversely proportional to the square roots of their formula masses. [It can be a ratio of molecular speeds, effusion/diffusion times, distance traveled by the molecules , or the amount of gas effused] Ptotal = P1 + P2 + P3 + ...Pn PV = nRT RateA = √molar massB RateB = √molar massA Abbreviations Atm = atmosphere mm Hg = millimeters of mercury Torr = another name for mm Hg or 1.33 Kpa Pa = Pascal kPa = kilopascal K = Kelvin 0C + 273 = K Standard Conditions 00C = 273 K 1.0 atm = 760.0 mm Hg = 101.325 kPa STP = 273k & 101.325 kPa R=0.0821 Latm/molK R=8.315 dm3kPa/molK Molecular vs Empirical Formula: Empirical is reduced to the lowest possible number for each coefficient if they are all reducible by the same amount. Law of Conservation of Mass: You end up with the same amount you started with, so equations have to be balanced ∆ = heat is added to an equation The ideal gas law is an important concept in chemistry. It can be used to predict the behavior of real gases in situations other than low temperatures or high pressures. This collection of ten chemistry test questions deals with the concepts introduced with the ideal gas laws. Useful information: At STP: pressure = 1 atm = 700 mmHg, temperature = 0 °C = 273 K At STP: 1 mole of gas occupies 22.4 L R = ideal gas constant = 0.0821 L·atm/mol·K or 8.3145cm3/mol·K Things you need to know: Reactions Types :use the letter method A,B,C,D Equations YOU should be able to do: put examples here to refer back to if needed Single replacement = Mole ratios: Double replacement = Synthesis or Combination = Decomposition = Stoichiometry: Combustion = Dimensional Analysis= example Moles to grams or grams to moles: Stoichiometry = example Representative Particles (RP) =amount Moles to liters or liters to moles: Gas Molecule Actions & Reactions = Moles to RP or RP to Moles: Kinetic Energy (KE) = vocabulary Atomic Particles and their functions: Boyle’s Law: Chemical Bonds form due to: Charles’ Law: Ions: Cation: Gay-Lussac’s Law: Anion: Isotopes: Electron Configurations: s, d, p, & f blocks: Combined Gas Law: Ionic Bonds: Dalton’s Law: Covalent Bonds: Ideal Gas Law: Diatomic Molecules: