Separation Technique stations Katharine and Alethea
advertisement
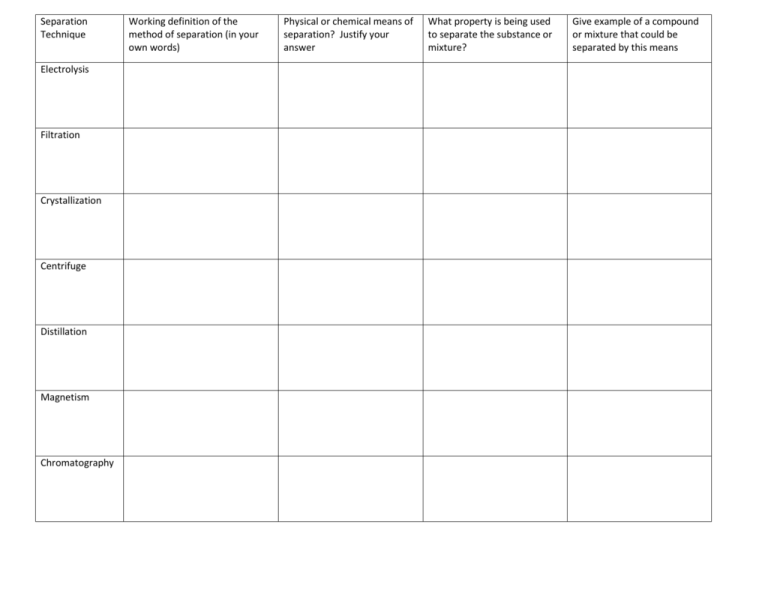
Separation Technique Electrolysis Filtration Crystallization Centrifuge Distillation Magnetism Chromatography Working definition of the method of separation (in your own words) Physical or chemical means of separation? Justify your answer What property is being used to separate the substance or mixture? Give example of a compound or mixture that could be separated by this means Drawings & Questions: Provide a drawing/description of what is happening at each station and answers to any questions Electrolysis Distillation Filtration Magnetism Crystallization Chromatography Centrifuge ELECTROLYSIS OF WATER Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water. Note: The hydrogen has a positive charge and is attracted the negative electrode. The oxygen has a negative charge and is attracted to the positive electrode. Questions: 1. Label in your drawing of this station where the hydrogen gas is and the oxygen gas is. 2. Why do you think there is more hydrogen gas than oxygen gas in the tubes? 3. Complete the following chemical equation for the decomposition of water: H2O Filtration and Crystallization 1. Put 1 scoop of the sand/salt mixture in a beaker. 2. Add about 50 mL of tap water. 3. Stir with stir rod to let the salt dissolve. 4. Fold your filter paper such that it fits in the funnel according to the diagram to the right: 5. Place a second beaker below the funnel and pour the sand/salt/water mixture into the funnel. 6. Once the water has gone through the filter, place the beaker with the saltwater (without the funnel) on a hot plate. 7. While you are waiting for the water to boil, fill out the chart for both filtration and crystallization. Be sure to write down observations before, during, and after of what happens to the salt water. 8. Take the filter paper out of the funnel and throw away. Centrifuge Centrifuges are devices or apparatus that can be used to separate insoluble materials (usually a solid) from a liquid, where normal filtration does not work well e.g. a suspension of very fine (tiny) solid particles. When spinning, suspended materials gets pushed to the bottom of the tube because the greater mass and greater inertia of the suspended materials. Centrifuging is widely used in biochemical labs for DNA and protein separation. CAUTION: The centrifuge consists of carriage or glass tube holder, mounted on an electrically motor driven vertical axle. The carriage holds the balanced glass tubes of equal amounts of the solid-liquid mixture in each tube, all tubes initially in a horizontal position before the motor is switched on. Instructions: 1. Fill one small test tube about ½ full of the muddy water. 2. Fill another small test tube with equal amount of distilled water. 3. Place the test tubes exactly opposite of each other in the centrifuge. Close the lid of the centrifuge tight and lock it. 4. If you have the teal and gray centrifuge, turn the timer to 2 minutes. If you have the yellow and silver centrifuge, turn the power button to “on” for about 2 minutes. 5. Do NOT open the lid until the centrifuge has stopped turning. Questions: 1. Describe what the muddy water looks like before and after the centrifuging. 2. Describe how the mud is separated from the water. Distillation Distillation involves 2 stages and both are physical state changes. Stage 1: The solution mixture is boiled to vaporize the most volatile liquid in the mixture. The most volatile liquid is the liquid that will evaporate from liquid to gas the fastest. Stage 2: The vapor is cooled by cold water in the condenser to condense the gas back to a liquid. The liquid is collected and is now called the distillate. Distillation can be used to purify water because the dissolved solids in water have a much higher boiling point and will not evaporate with the steam. Questions: 1. Write a definition for volatile. 2. Which liquid has the higher boiling point: the alcohol or the colored water? Defend your answer. Magnetism Magnetism can be used to separate magnetic materials, such as iron, from nonmagnetic materials. Magnets are used in recycling to recover iron and steel from domestic waste. “Rubbish” is on a conveyer belt that passes under a powerful magnet which pluck’s out magnetic materials. Procedure Do NOT remove the magnet from the bag. 1. Hold the magnet under the Petri dish with sulfur in it. 2. Hold the magnet under the Petri dish with iron in it. 3. Hold the magnet under the Petri dish with the sulfur/iron mixture. Question: 1. Write down other ways magnets are used or could be used in daily life (example: magnetic jewelry clasps). PAPER CHROMOTOGRAPHY This method of separation is used to separate particles of different size. It is especially used to separate pigments or different dyes in food coloring or markers. The material to be separated for example, a food dye, is dissolved in a solvent and carefully spotted onto chromatography paper. The paper is carefully dipped into a solvent, which is absorbed into the paper and rises up it. The solvent may be water or an organic liquid like an alcohol (ethanol). The smaller molecules move up the paper farther because it easier to move through the paper. PROCEDURE: 1. Your group needs to select three different black pens or markers and do a separate strip in a separate test tube for each marker. (3 papers total) 2. Place a dot of ink about 2 cm from the bottom of the chromatography paper. 3. Use the distilled water bottle to fill the test tube to 1 cm deep. 4. Place the chromatography paper into the test tube. Be certain that the ink dot is ABOVE the water line. 5. Watch the water travel up the chromatography paper separating the colors and record your observation. 6. While you are waiting look at the examples that have already been done and answer the question Questions 1. Of the colors in the black dye which color is made of the largest molecules? Justify your answer. ELECTROLYSIS OF WATER Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water. Note: The green color in this case represents a neutral pH. The hydrogen has a positive charge and is attracted the negative electrode and because hydrogen makes the water acidic, the solution turns color (in this case, yellow). The oxygen has a negative charge and is attracted to the positive electrode and makes the water basic, turning the solution to blue. Questions: 1. Label in your drawing of this station where the hydrogen gas is and the oxygen gas is. 2. Observe which tube has more gas being produced. This is the hydrogen gas. Why does it make sense that there should be more hydrogen than oxygen? 3. Complete the following chemical equation for the decomposition of water: H2O







