Lab 12-dna
advertisement

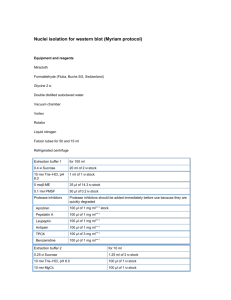
Lab 13 DNA Extraction and PCR DNA Extraction – Qiagen DNeasy Plant Mini Kit 1. Using a Mortar & Pestle, grind the tissue in a small quantity of Liquid Nitrogen. Pour tissue into a sterile 2ml tube. 2. Add 400uL Buffer AP1 and 4uL RNase A. Vortex and incubate for 10minutes at 65C. Invert your tube 2-3 times during the incubation. 3. Add 130uL Buffer P3. Mix and incubate on ice 5 minutes. 4. Centrifuge at 14,000RPM for 5 minutes. 5. Pipet lysate into a QIAshredder spin column placed in a 2mll collection tube. 6. Centrifuge at 14,000PRM for 2 minutes. 7. Transfer flow-through into a new 2ml tube without disturbing pellet. Add 1.5 volumes of Buffer AW1. Mix by pipetting. 8. Transfer 650uL of mixture into DNeasy Mini spin column placed in a 2ml collection tube. Centrifuge at 8000RPM for 1 minute. Discard flow-through. Repeat for remaining sample. 9. Place spin column into a new 2ml collection tube. Add 500uL Buffer AW2. Centrifuge at 14,000RPM for 1 minute. Discard flow-through. 10. Add another 500uL Buffer AW2. Centrifuge at 14,000RPM for 1 minute. Discard flow-through. 11. Place spin column into a new 1.5ml Centrifuge tube. 12. Add 50uL Buffer AE (warm AE at 65C before use). Incubate at room temperature for 5 minutes. 13. Centrifuge 8000RPM for 1 minute. 14. Repeat 12 & 13 PCR: Each pair of students will prepare one tube of MasterMix, enough for 3 reactions (one for each student and a negative control). The two primers to be used are: FW: FT-ATG – a primer that amplifies at the start codon of both the FT1 and FT3 genes Sequence: atgtctagcagggagagagaccctc RVRS: FT-MIDSTOP – an internal primer that amplifies in an intronic region ~600 bp downstream of the start codon in both FT1 and FT3 Sequence: attccaaggtgatcaatggcacagtg MasterMix Recipe Master Mix Recipe to individual reaction tubes add: 5x green go taq buffer 22 mM MgCl2 5 mM dNTPs Primer 1 100 ng/µl Primer 2 100 ng/µl Taq polymerase Sterile H2O per rxn (µl) 10 5 1 0.5 0.5 0.25 31.75 1:50 dilution DNA Total 1 50 per 3 rxns (µl) 30 15 3 1.5 1.5 0.75 95.25 Cycling Parameters: 95° - 2 min 95° - 30 sec 66° - 30 sec 72° - 45 sec - the extension time is determined by the length of the region being amplified, 1 minute per kb 72° - 5 min 4° - infinite 40 cycles - the number of cycles is the number of times that steps 2-4 are repeated The PCR products will be saved for next week’s lab.










![mRNA Purification Protocol [doc]](http://s3.studylib.net/store/data/006764208_1-98bf6d11a4fd136cb64d21a417b86a59-300x300.png)