Observing Chemical Change
advertisement

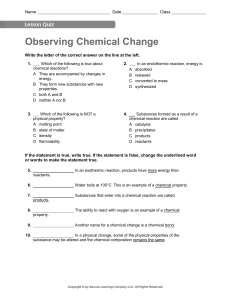
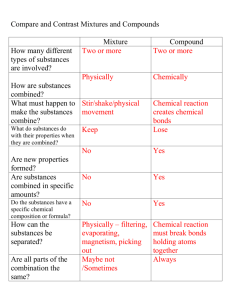
Observing Chemical Change • Matter is anything that has mass and takes up space. • Chemistry is the study of matter and how matter changes. • Changes in matter can be described in terms of physical changes and chemical changes. Properties of Matter • PHYSICAL PROPERTIES: Properties of the substance that can be observed without changing the substance to another substance. Example: Color, shape, size, melting point, ability to conduct heat and electrical current………… • CHEMICAL PROPERTIES: Properties of a substance that describes its ability to change into other substances. Example: Does it react? Or it does not react? Is it flammable? Or it is not flammable? Changes of Matter • Physical Change: Changes that change the form or the appearance of the substance but does not make the substance into another substance. • Chemical change: Is a change in matter that produces one or more new substances, Chemical change is also known as Chemical Reaction. In Chemical reactions two or more substances known as reactants undergo change and form new substances known as products • Chemical Change occurs when bonds break and new bonds form Evidence for Chemical Change • Chemical reactions involve changes in properties and changes in energy that you can observe. • Evidence, (Clues) for chemical reaction: • • • • Solid formation (precipitate) Gas formation Color change Energy change Changes in energy • Endothermic Reaction: Chemical reactions where energy is taken in. • Exothermic Reaction: Chemical reactions where energy is given off.