DNA Extraction for fungi ()
advertisement

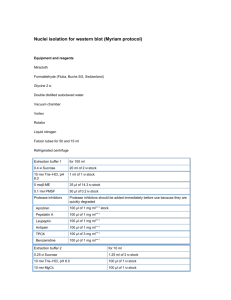
DNA Extraction for fungi. 1. Prepare 2 sets of tubes and label. 1 set add 0.5ml (500μl) EXTRACTION BUFFER 1 set add 0.3ml (300μl) Isopronanol 2. Collect approx. 20-70 mg of fungal mycelia with toothpick/plastic tip and put into EXTRACTION BUFFER. (approx 1.5 cm2 = 60 mg) This is easier when the mycelium has been growing on cellophane first. (Avoid keeping mycelia in EXTRACTION BUFFER for long periods.) 3. Pulverize the fungal mass in the EXTRACTION BUFFER for 1-2 seconds with the machine. Push the mycelial tissue with the plastic pestle. WASH tip between samples, dip pestle into 70% ethanol and operate for 1-2 seconds. Dry pestle with a wipe (Drying is not required). Do not push pestle against tube or operate for more than 5 seconds. 4. CENTRIFUGE cell lysate at 5,000 rpm for 10 minutes. DECANT supernatant directly into tubes containing 0.3ml (300μl) Isopronanol. DISCARD remaining lysate (~0.1ml) and cell debris after decanting. 5. Mix LYSATE and ISOPROPANOL by inverting tube several times. CENTRIFUGE tube at 12,000 rpm for 10 minutes DISCARD supernatant as much as possible. (Wash tube with 0.8ml of 70% ethanol OPTIONAL.) 6. EVAPORATE remaining alcohol by incubation at 37°c for 15 minutes. Speed vac can be used for drying. ADD 50μl of distilled water and RNase and dissolve DNA pellet by tapping or vortexing at low speed. EXTRACTION BUFFER: 7.45 g 10 ml 2 ml Potassium Chloride 1 M Tris-HCl (pH 8.0) 0.5 M EDTA (pH 8.0) Make solution up to 100 ml with distilled water. Sterilization is only required for long term storage.