Gas Laws - Teach.Chem
advertisement
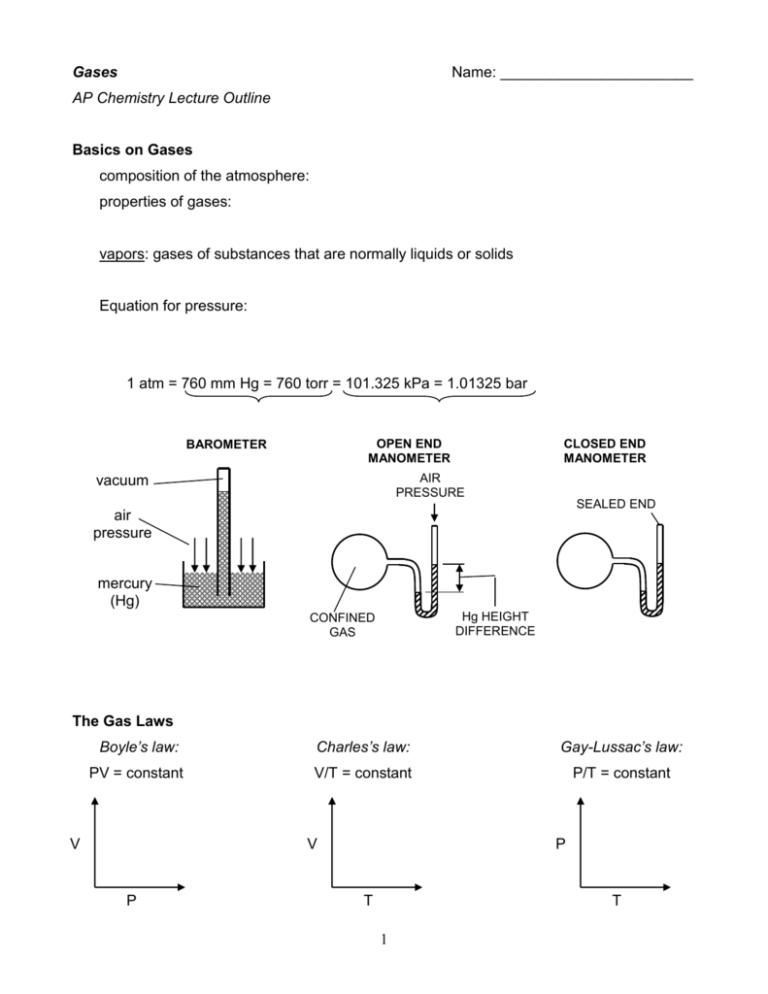
Gases Name: _______________________ AP Chemistry Lecture Outline Basics on Gases composition of the atmosphere: properties of gases: vapors: gases of substances that are normally liquids or solids Equation for pressure: 1 atm = 760 mm Hg = 760 torr = 101.325 kPa = 1.01325 bar OPEN END MANOMETER BAROMETER CLOSED END MANOMETER AIR PRESSURE vacuum SEALED END air pressure mercury (Hg) Hg HEIGHT DIFFERENCE CONFINED GAS The Gas Laws Boyle’s law: Charles’s law: Gay-Lussac’s law: PV = constant V/T = constant P/T = constant V V P P T T 1 Avogadro’s law: Equal volumes of gas at the same temperature and pressure have the same number of particles. Combined Gas law: merges Boyle’s, Charles’s, and Gay-Lussac’s laws in one equation. **NOTE: For all gas law calculations, use the absolute temperature (in K). Ideal Gas law: n = number of moles of gas R = 8.314 L-kPa/mol-K = 0.08206 L-atm/mol-K Conditions of standard pressure and temperature (STP): Equation for gas density (in g/L): d P RT = molar mass of gas To solve problems involving volumes of gases in chemical reactions: 1. Use PV = nRT to get any and all gases in terms of moles (i.e., n). 2. Solve the problem using stoichiometry. EX. Safety air bags in automobiles are inflated by nitrogen gas generated by the rapid decomposition of sodium azide, according to 2 NaN3(s) 2 Na(s) + 3 N2(g) If an air bag has a volume of 36 L and is to be filled with nitrogen at 1.15 atm and 26.0oC, how many grams of sodium azide must be decomposed? 2 Dalton’s law of Partial Pressure Ptot = P1 + P2 + ... total pressure of mixture partial pressures: Other equations: Total moles of gas in a mixture of gases can be found using: The mole fraction (X) of a gas in a mixture is found using: EX. Find the total pressure exerted by 14 g of carbon monoxide and 14 g of nitrogen in a 6.0-L container at 25oC. Collecting Gases over Water gas being collected filled with water, initially collected gas (w/H2O vapor, too) gas from reaction H2O levels even before reaction during reaction reaction complete After the reaction is complete, raise or lower the collecting vessel so that the water levels inside and out are the same. In this way... 3 EX. For the reaction CaC2(s) + H2O(l) C2H2(g) + CaO(s)... If 0.852 L of acetylene are collected over water at 20.0 oC, find the number of moles of acetylene collected and the number of grams of calcium carbide used. Assume the barometric pressure to be 732.0 torr. EX. Find the total pressure in container Z. PX A B C 1.3 L 3.2 atm 2.6 L 1.4 atm 3.8 L 2.7 atm VX VZ Z 2.3 L X atm PX,Z A B C The Kinetic-Molecular Theory (the theory of moving molecules) 1. Gas particles are in constant, random motion. 2. The volume of the particles is negligible compared to the container volume. 3. The attractive-repulsive forces between particles are negligible. 4. Collisions are perfectly elastic (i.e., no energy is lost). 5. KEavg of molecules is proportional to absolute temperature. At a given temperature, the gas particles of Sample A have the same KE as the gas particles of Sample B. 4 pressure = “how hard” and “how often” gas particles collide with the sides of the container Particle-Velocity Distribution (various gases, same T and P) # of particles Particle-Velocity Distribution (same gas, same P, various T) # of particles (SLOW) Velocity of particles (m/s) (FAST) (SLOW) Velocity of particles (m/s) (FAST) Equation for the rms (root-mean-square) speed of a gas: R = 8.314 J/mol-K = molar mass, in kg KEavg for a particle = EX. Find the rms velocity of chlorine gas at 80oC. effusion: the escape of gas particles through a tiny hole into an evacuated space diffusion: the spread of one substance from high concentration to low concentration 5 For gases, rates of diffusion & effusion obey Graham’s law: The rate of diffusion of gases is slower than the molecular speeds because of... -The mean free path is the average distance traveled by a particle between collisions. -- it is shorter when the pressure is high EX. At a given temperature, an unknown noble gas travels 1.26 times faster than oxygen gas. What is the noble gas? Real Gases: Deviations from Ideal Behavior All real gases deviate, to some degree, from PV = nRT. The deviations are most pronounced at... Real gas particles... (1) (2) ideal gas (ideal gas equation): real gas (van der Waals equation): PV nRT P P nRT V nRT n2a 2 V - nb V The constants a and b are unique for each gas. -- a is large when interparticle attractions are large -- b is large for large gas particles 6