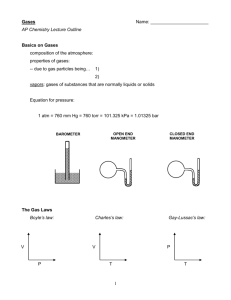
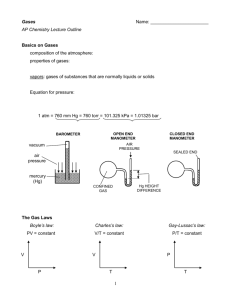
PROPERTIES OF GASES
advertisement

SECTION 14.1 LARA BEKARIAN QUARTER 4 CHEMISTRY TECHNOLOGY PROJECT MRS. JESSEN MAY 20, 2013 SECTION 14.1 PROPERTIES OF GASES OBJECTIVES • Learn about compressibility. • Learn factors that affect gas pressure. • Learn kinetic theory. • Learn why gases are easier to compress than solids or liquids. SECTION 14.1 COMPRESSIBILITY Compressibility is a measure of how much the volume of matter decreases under pressure. Gases are easily compressed, or squeezed into a smaller volume. Example: A car collision with an inflated air bag cause much less damage than a collision with a steering wheel or dashboard because when a person collides with an inflated air bag, the impact forces the molecules of gas in the bag closer together, the compression of the gas absorbs the energy of the impact. SECTION 14.1 FACTORS AFFECTING GAS PRESSURE The factors that affect gas pressure are; the amount of gas, volume, and temperature. Example (amount of gas): When gas is pumped into a container, the pressure increases as more particles are added. If the number of particles double, the pressure doubles. Example (volume): A piston can be used to force a gas in a cylinder into a smaller volume. When the volume is decreased, the pressure the gas exerts is increased. Example (temperature): An increase in temperature causes an increase in pressure of an enclosed gas. The container can explode if there is too much of an increase in the pressure. SECTION 14.1 KENETIC ENERGY Kinetic molecular theory: the body of theory that explains the physical properties of matter in terms of the motions of its constituent particles. Gases are composed of a large number of particles that behave like hard, spherical objects in a state of constant, random motion. These particles move in a straight line until they collide with another particle or the walls of the container. These particles are much smaller than the distance between particles. Most of the volume of a gas is therefore empty space. There is no force of attraction between gas particles or between the particles and the walls of the container. Collisions between gas particles or collisions with the walls of the container are perfectly elastic. None of the energy of a gas particle is lost when it collides with another particle or with the walls of the container. SECTION 14.1 GASES ARE EASIER TO COMPRESS THAN SOLIDS OR LIQUIDS Gases are easily compressed because of the space between the particles. The distance between particles in gas is much greater than the distance between particles in a liquid or solid, making gas easier to compress. THANKYOU SECTION 14.1 CITED SOURCES http://www.cs.cmu.edu/~rapidproto/csc/compress.html http://chemed.chem.purdue.edu/genchem/topicreview /bp/ch4/kinetic4.html http://www.ucolick.org/~bolte/AY4_00/week6/sun_fusion .html