Answers for Study Guide for Chemistry Test I
advertisement

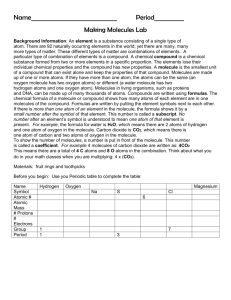
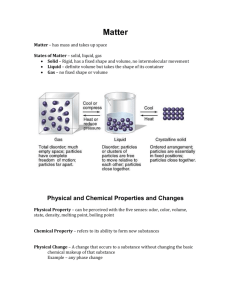
Answers for Study Guide for Chemistry Test (Atoms, Elements, Compounds, & Mixtures) 1. What is matter? Anything that has mass and takes up space (volume) 2. What is an atom? An extremely small particle of all matter 3. What are the 2 parts of the atom’s nucleus? the proton and neutron 4. What tells you the number of protons in the nucleus of each element? Atomic Number 5. What part of the atom has a negative charge and where is it found? Electron; found in the energy clouds that surround the nucleus of the atom 6. How do you find the number of neutrons in the nucleus of a neutral atom? You must round off the atomic mass to a whole number and subtract the atomic number from the rounded atomic mass. 7. What part of the atom has a positive charge and where is it found? Proton; found in the nucleus 8. How many electrons can be in energy cloud 2? There can be up to 8 electrons in this cloud. 9. What is a pure substance made of only one kind of atom and what do you know about them? Element; they are pure; they are listed on the Periodic Table; there are about 100 of them that are found naturally on earth; they cannot be changed into any other substance. 10. What is a pure substance made of two or more elements chemically combined and what do you know about them? Compounds; they are pure; they are made when 2 or more atoms chemically bond to form the molecule; a compound is made of the same molecule throughout; a chemical formula will tell you the types of elements and the number of atoms that are bonded together to form the molecule. 11. What is the smallest unit of a compound that still has the properties of the compound? molecule 12. What are substances that are just mixed together?mixtures 13. What are the two types of mixtures? Homogeneous and heterogeneous 14. Which type of mixture can you see the individual parts? Heterogeneous 15.How can you separate a heterogeneous mixture with large size particles that you easily seeYoucan sort them using your eyes 16. When will a magnet help you separate a mixture? This works when one substance is attracted by the magnet and the other one is not. 17. What is H2O and what elements are in this compound? Water; there are 2 elements: Hydrogen and Oxygen 18. What is the formula for carbon dioxide? CO2 19. What is the symbol for oxygen? O 20. What is the name for this symbol: Na? Sodium 21.Give the scientific and common name of this: NaCl. Sodium Chloride or Table salt; there is one atom of Sodium and one atom of Chlorine in this molecule. That means that there are 2 atoms in this molecule. 22. How can you separate compounds? You must break the bonds between the atoms to separate a compound 23. What does a subscript tell you about the element? The subscript tells you how many atoms there are of that specific element. 24. What is a subscript? The lower number on a compound formula that tells you how many atoms of that element is found in the compound’s molecule 25. Label these as an element; compound; or mixture: Air- mixtureWater- compoundCopper- elementSalt- compound Oxygen- element Gold- elementVinegar- compound Ice cream- mixture