Bonding.VSEPR.Molecular Modeling.01
advertisement
Chemistry
LESSON:
VSEPR Theory 1
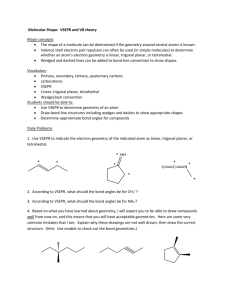
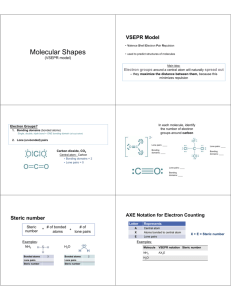
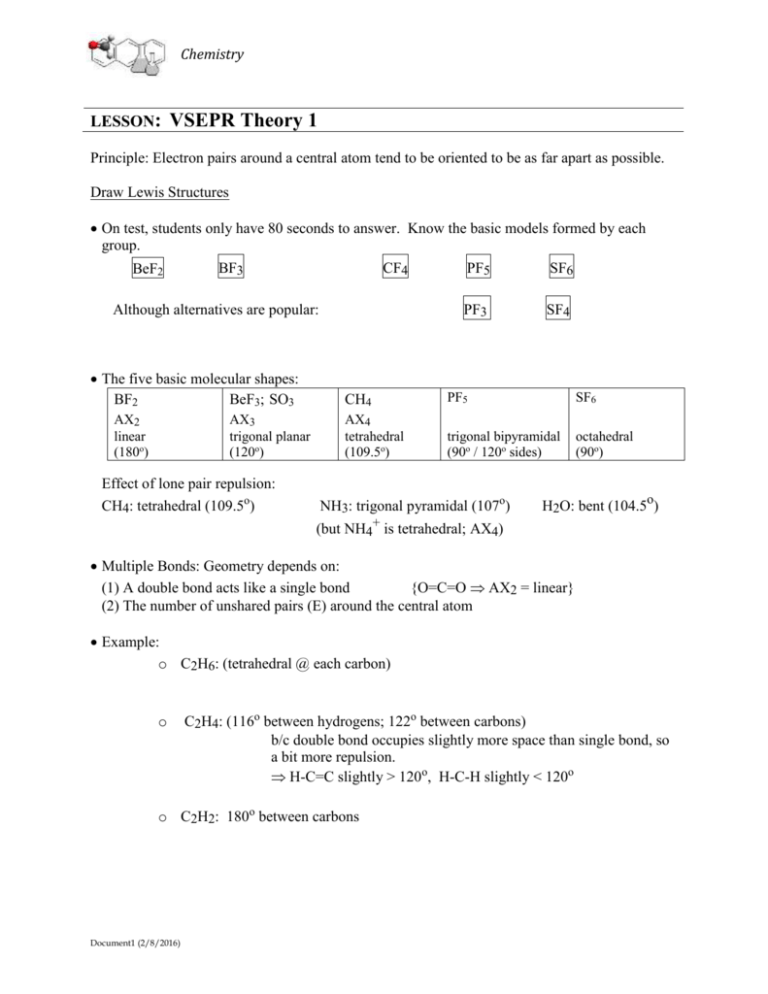
Principle: Electron pairs around a central atom tend to be oriented to be as far apart as possible.
Draw Lewis Structures
On test, students only have 80 seconds to answer. Know the basic models formed by each
group.
BF3
CF4
PF5
SF6
BeF2
Although alternatives are popular:
The five basic molecular shapes:
BF2
BeF3; SO3
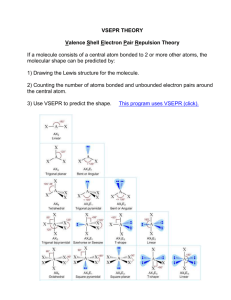
AX2
linear
(180o)
AX3
trigonal planar
(120o)
Effect of lone pair repulsion:
CH4: tetrahedral (109.5o)
PF3
SF4
CH4
PF5
SF6
AX4
tetrahedral
(109.5o)
trigonal bipyramidal
(90o / 120o sides)
octahedral
(90o)
NH3: trigonal pyramidal (107o)
H2O: bent (104.5o)
(but NH4+ is tetrahedral; AX4)
Multiple Bonds: Geometry depends on:
(1) A double bond acts like a single bond
{O=C=O AX2 = linear}
(2) The number of unshared pairs (E) around the central atom
Example:
o C2H6: (tetrahedral @ each carbon)
o
C2H4: (116o between hydrogens; 122o between carbons)
b/c double bond occupies slightly more space than single bond, so
a bit more repulsion.
H-C=C slightly > 120o, H-C-H slightly < 120o
o C2H2: 180o between carbons
Document1 (2/8/2016)
Chemistry
Molecular Modeling
VSEPR & Molecular Modeling
p. 2
Atom....................Color
carbon..................black
oxygen .................. red
nitrogen ...............blue
hydrogen ...........white
halogens ............green
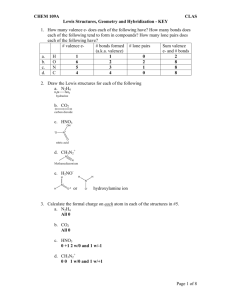
Draw Lewis Structures & Make Models for:
H2
Lewis Structure
CH4
NH3
H2O
VSEPR Shape
(e.g., AX2)
Geometry
Bond Angle
(modification
from lone pairs)
CH3OH
Lewis Structure
VSEPR Shape
(e.g., AX2)
Geometry
Bond Angle
(modification
from lone pairs)
H3C-CHOH-CH3
Chemistry
VSEPR & Molecular Modeling
p. 3
Hydrocarbons – Alkane Series
C2H6
C2H4
C2H2
Lewis Structure
VSEPR Shape
(e.g., AX2)
Geometry
Bond Angle
(modification
from lone pairs)
C2 doesn’t exist. Why?
Hydrocarbons – Isomers (same chemical formulas, different structures)
C2H2Cl2
C3H8O
(two isomers)
(two isomers)
Lewis Structure
VSEPR Shapes
(e.g., AX2)
Geometry
Bond Angle
(modification
from lone pairs)
C3H6
(straight chain & ring)
Chemistry
VSEPR & Molecular Modeling
p. 4
Example of Isomers: One flavor in basil (anethole) is an isomer of another chemical found in
anise (estragole).