VSEPR Worksheet
1)
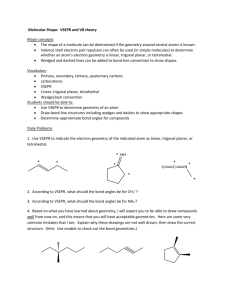
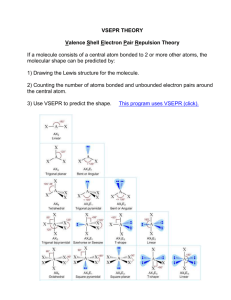
Briefly describe the primary ideas behind VSEPR theory.
2)
For each of the following compounds, a Lewis structure, determine the
bond angles and molecular shapes for all atoms:
a)
BI3
b)
CH4
c)
NF3
d)
C2H2
e)
SCl6
VSEPR Worksheet - Solutions
1)
Briefly describe the primary ideas behind VSEPR theory.
Electrons are negatively charged and like repels like so the atoms
in a molecule will likely be as far away from each other as
possible.
2)
For each of the following compounds, draw a Lewis structure, determine
the bond angles and molecular shapes for all atoms:
a)
BI3
120o
..
..
I
..
..
B
I
..
..
..
120o
..
I
..
b)
CH4
120o
trigonal planar
H
109.5o
H C H
H
c)
109.5o
tetrahedral
NF3
107.5o
F
..
..
..
107.5o
..
..
..
..
F
.. ..
F
N ..
107.5o
trigonal pyramidal
Everett Community College does not discriminate on the basis of race, religion, creed, color, national origin, age, sex,
marital status, disability, or veteran status.
d)
C2H2
180o
H C C H
e)
SCl6
..
Cl
..
Cl
Cl
..
90 ..
S
Cl
Cl
..
Cl
..
..
..
90o
..
..
..
..
..
....
..
..
o
octahedral
..
Everett Community College does not discriminate on the basis of race, religion, creed, color, national origin, age, sex,
marital status, disability, or veteran status.