C3 3 energy calculations checklist
advertisement
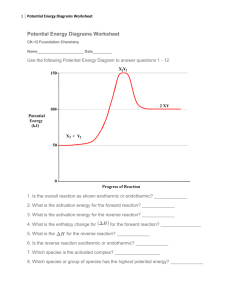
Topic: C3 3 Energy calculations Date Specification Content C3.3 Calculating and explaining energy change Knowing the amount of energy involved in chemical reactions is useful so that resources are used efficiently and economically. It is possible to measure the amount of energy experimentally or to calculate it. Candidates should use their skills, knowledge and understanding to: Consider the social, economic and environmental consequences of using fuels. Interpret simple energy level diagrams in terms of bond breaking and bond formation (including the idea of activation energy and the effect on this of catalysts). Evaluate the use of hydrogen to power cars compared to other fuels C3.3.1 Energy from reactions a) The relative amounts of energy released when substances burn can be measured by simple calorimetry, e.g. by heating water in a glass or metal container. This method can be used to compare the amount of energy released by fuels and foods. You should be able to calculate and compare the amount of energy released by different fuels given the equation: Q = mc T b) Energy is normally measured in joules (J). c) The amount of energy released or absorbed by a chemical reaction in solution can be calculated from the measured temperature change of the solution when the reagents are mixed in an insulated container. This method can be used for reactions of solids with water or for neutralisation reactions. d) Simple energy level diagrams can be used to show the relative energies of reactants and products, the activation energy and the overall energy change of a reaction. You will be expected to understand simple energy level diagrams showing the relative energies of reactants and products, the activation energy and the overall energy change, with a curved arrow to show the energy as the reaction proceeds. You should be able to relate these to exothermic and endothermic reactions. e) During a chemical reaction: Energy must be supplied to break bonds. Energy is released when bonds are formed. Darwen Vale High School Science Department 2013 Name: Comments 1 Topic: C3 3 Energy calculations Date f) g) h) i) Specification Content In an exothermic reaction, the energy released from forming new bonds is greater than the energy needed to break existing bonds. (HT only) You should be able to calculate the energy transferred in reactions using supplied bond energies. (HT only) In an endothermic reaction, the energy needed to break existing bonds is greater than the energy released from forming new bonds. (HT only) Catalysts provide a different pathway for a chemical reaction that has a lower activation energy. You should be able to represent the effect of a catalyst on an energy level diagram. Hydrogen can be burned as a fuel in combustion engines. Name: Comments hydrogen + oxygen water It can also be used in fuel cells that produce electricity to power vehicles. You should be able to compare the advantages and disadvantages of the combustion of hydrogen with the use of hydrogen fuel cells from information that is provided. Darwen Vale High School Science Department 2013 2