Practice Quiz on Water: Answer Key
advertisement

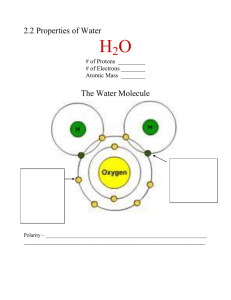
FORMATIVE ASSESSMENT: PROPERTIES OF WATER/pH scale Name: ________________________________ Teacher_____________________Date: _____________________ Learning Goals: Students will be able to: Understand how water supports life. Understand the pH scale and how relates to acids and bases. Score 2: 1. Water droplets form by water molecules being attracted to each other. What term best explains this behavior? a. adhesion c. solubility b. cohesion d. magnetism 2. Which property of water allows water to stick to the sides of the straw? a. adhesion c. heat capacity b. cohesion d. solubility 3. Which property of water allows bugs to walk on water? a. adhesion b. heat capacity c. surface tension d. solubility 4. Water takes _______ heat to change to its gaseous state. a. large amounts of b. small amounts of c. no 5. What property of water allows sweat to cool the body & coastal cities to have more moderate temperatures than inland cities? a. High heat capacity c. Surface tension and capillary action b. Neutral pH of 7 d. Universal solvent 6. A solution with a pH of 3 is: a. acidic b. basic c. neutral 7. A solution with a pH of 8 is: a. acidic b. basic c. neutral Score 3: 8. Water is a polar molecule because a. It shares electrons equally between oxygen and hydrogen b. One side of the molecule has a slight positive charge and the other side has a slight negative charge c. It freezes at zero degrees Celsius d. It forms hydrogen bonds with other water molecules 9. Which side of the water molecule has a slight negative charge? a. oxygen side b. hydrogen side FORMATIVE ASSESSMENT: PROPERTIES OF WATER/pH scale 10. What type of bond exists where the arrows are pointing? a. ionic b. covalent c. hydrogen d. metallic 11. Water molecules form what type of bonds with each other, which is depicted in the diagram to the right. a. ionic c. hydrogen b. covalent d. metallic 12. Surface tension and capillary action occur in water because it a. is very dense b. is nonpolar c. has ionic bonds d. has hydrogen bonds 13. What kinds of substance can dissolve in water? a. nonpolar molecules b. polar molecules c. both 14. Which of the following is true for a neutral solution? a. H+ is greater than OHb. OH- is greater than H+ c. H+ is equal to OH- 15. Which of the following is considered a basic solution? a. H+ is greater than OHb. OH- is greater than H+ c. H+ is equal to OH-