pola27425-sup-0001-suppinfo01
advertisement

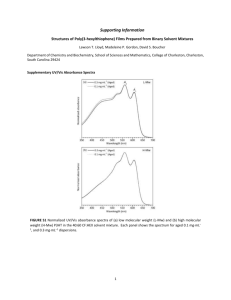
Synthesis of 2,5-dibromo-3-hexylthiophene [1] Magnesium turnings (5.75 g, 0.237 mol) and diethyl ether (125 mL) were added to a dry 250 mL Schlenk flask and placed in an ice bath. Bromohexane (31.56 g, 0.191 mol) was slowly added and the solution was stirred for 2 h under N2 gas. The solution was transferred to an addition funnel fitted to a flask containing 3-bromothiophene (25 g, 0.153 mol), 1,3-bis(diphenylphosphino)propane nickel(II) dichloride (Ni(dppp)Cl2) (0.43 g, 0.001 mol), and 60 mL of diethyl ether. The flask was cooled to 0 oC and the Grignard reagent was added dropwise. The reaction was stirred at room temperature under N2 for 3 days. The reaction mixture was slowly added to dilute HCl solution, washed 3 times with water, dried with sodium sulfate and filtered. The solvent was removed and the crude product was distilled to produce a colorless liquid. The purified product (25 g, 0.149 mol) was added to a dried 500 mL Schlenk flask with NBS (50 g, 0.281 mol), acetic acid (200 mL) and DCM (200 mL). The solution stirred for 18 h under N2. The reaction mixture was washed 5 times with water, 5 times with saturated sodium bicarbonate, dried over sodium sulfate, and filtered. The solvent was removed and the resulting oil was further purified by column chromatography with hexanes as an eluent and then distilled twice to produce a yellowish oil. (32g, 65%) 1H NMR (500 MHz, CDCl3): 6.8 (s, 1H), 2.5 (t, 2H), 1.6 (m, 2H), 1.21.4 (m, 6H), 0.92 (t, 3H). Scheme S1. Polymerization of tert-butylacrylate with P3HT-DATC Figure S1. 1H NMR of P3HT-b-PtBA 4hrs. Molecular weight determination of P3HT-b-PtBA. To determine the mole ratio of poly(tertbutylacrylate) to P3HT, the methine proton b ( 2.40 – 2.10) of the 1H NMR was integrated to the methylene protons closest to the thiophene ring of the hexyl group a ( 3.00 – 2.55). The result is the amount of tert-butylacrylate (tBA) repeat units per 3HT repeat unit. This number multiplied by the DP of P3HT gives the DP of the PtBA and thus a total molecular weight. For P3HT-b-PtBA 4hrs in Figure S5: 1.94 tBA per 3HT (mole fraction of 0.66 PtBA to 0.34 P3HT). For P3HT DP = 49, PtBA DP = 49*1.94 = 95. So the composition is P3HT49-b-PtBA95 and Mn = 20200 g/mol. Scheme S2. Polymerization of 4-vinylpyridine with P3HT-DATC Figure S2. 1H NMR of P3HT-b-P4VP 36 hrs. Molecular weight determination of P3HT-b-PtBA. To determine the mole ratio of poly(4-vinylpyridine) (P4VP) to P3HT from the 1H NMR, the proton on the thiophene ring b ( 7.10 – 6.85) was integrated to two aromatic protons c ( 8.90 – 8.00). The result is the amount of 3HT repeat units per 4vinylpryidine (4VP) repeat unit. This number and the DP of P3HT gives the DP of the PtBA and thus a total molecular weight. For P3HT-b-P4VP 36 hrs in Figure S2: 0.53 3HT per 4VP (mole fraction of 0.65 P4VP to 0.34 P3HT). For P3HT DP = 20, P4VP DP = 20*(1/0.53) = 20*1.89 = 38. So the composition is P3HT20-b-P4VP38 and Mn = 8200 g/mol. 1. Mougnier, S.J., et al., Design of well-defined monofunctionalized poly(3-hexylthiophene)s: toward the synthesis of semiconducting graft copolymers. Macromolecular Rapid Communications, 2012. 33(8): p. 703-9.