Name Period ______ Chapter 10: The Mole Keller/Rosenzweig
advertisement

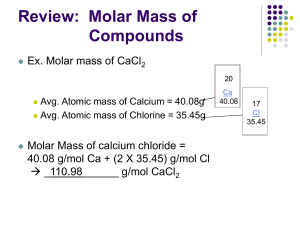
Name ____________________________________________________________ Period ____________ Chapter 10: The Mole Keller/Rosenzweig Mole Conversions with Compounds Once the molar mass of a compound is calculated, it can be used for mole conversions in the same way as atomic mass, using the conversion factors. Use the example below as a guide if needed. Example: Convert 2.45g of chromium (III) cyanide to moles STEP 1: Write the correct formula. Since chromium (III) cyanide is ionic, charges will need to be crossed. Cr+3 CN-1 = Cr(CN)3 STEP 2: Find the molar mass: 1 atom Cr (52.0g/mol) + 3 atoms C (12.0g/mol) + 3 atoms N (14.0g/mol) = 130.0g Cr(CN)3 1 mol Cr(CN)3 STEP 3: Use the molar mass for dimensional analysis: 2.45g Cr(CN)3 1 mol Cr(CN)3 = 0.018885 mol Cr(CN)3 130.0g Cr(CN)3 STEP 4: Go back to the original problem to determine the number of significant figures to include in the answer. 2.45g = 3 significant digits; ANSWER: 0.0189 mole Cr(CN)3 PRATICE PROBLEMS: 1. Find the mass of 0.655 moles of potassium phosphate. potassium hydroxide: _______________________ 2. How many moles are in 35.0g of aluminum hydroxide? aluminum hydroxide: _______________________ 3. Find the mass of 1.25 moles of trisilicon tetranitride. trisilicon tetranitride: ________________________ 4. How many molecules are in 14.0g of pentacarbon decahydride? pentacarbon decahydride: __________________ 5. Find the mass of 2.25 x 1022 formula units of magnesium cyanide. magnesium cyanide: ____________________ 6. How many molecules are in 14.0g of hydronitric acid? hydronitric acid: _____________ 7. How many molecules are in 25.3g of carbon dioxide? carbon dioxide: ____________