CHEM 2115
advertisement
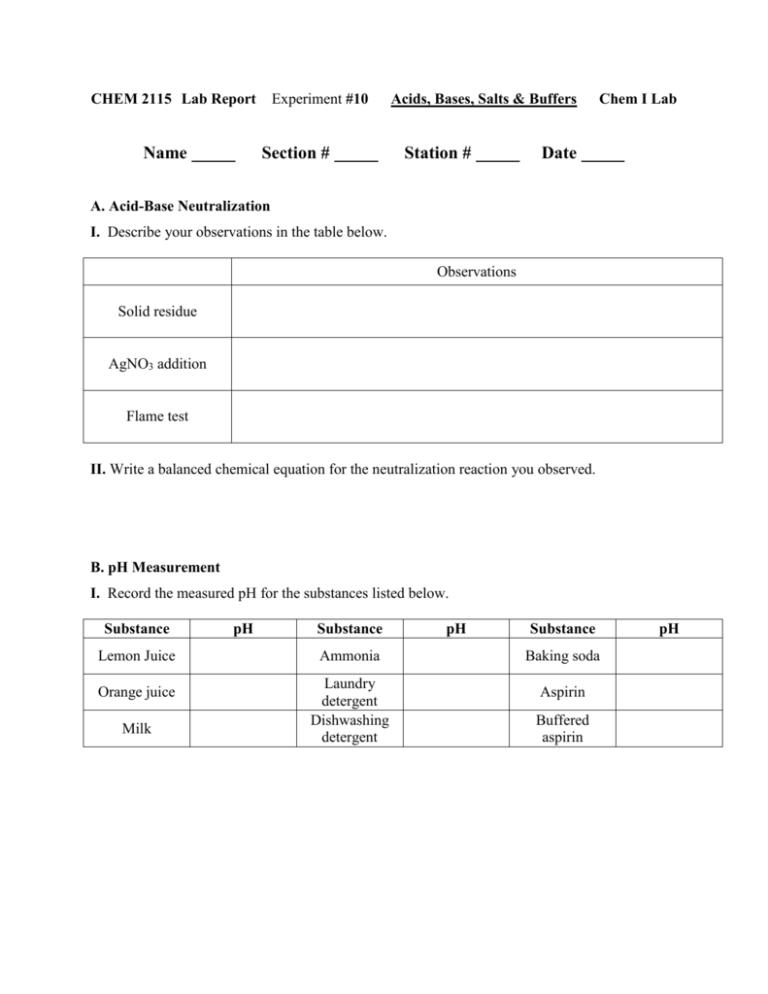
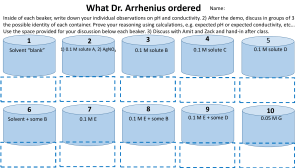
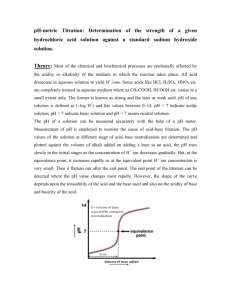
CHEM 2115 Lab Report Name Experiment #10 Section # Acids, Bases, Salts & Buffers Station # Chem I Lab Date A. Acid-Base Neutralization I. Describe your observations in the table below. Observations Solid residue AgNO3 addition Flame test II. Write a balanced chemical equation for the neutralization reaction you observed. B. pH Measurement I. Record the measured pH for the substances listed below. Substance Lemon Juice Orange juice Milk pH Substance Ammonia Laundry detergent Dishwashing detergent pH Substance Baking soda Aspirin Buffered aspirin pH C. Acid-Base Indicators Indicator Color in 0.05 M HCl Color in 0.05 M NaOH Methyl red Bromcresol green Phenolphthalein Methyl orange Methyl violet I. Which indicator would be best in distinguishing between 0.05 M HCl and 0.05 M NaOH? Which would be the worst? Justify your answer. D. Behavior of Salt Solutions Salt Solution pH Sodium acetate Sodium carbonate Ammonium chloride Sodium chloride Boiled distilled water I. Which ions exhibit significant Brønsted acid character? Brønsted base character? Are your results consistent the expected results based on the theory of hydrolysis? E. Buffers Buffer Initial pH pH after adding HCl pH after adding NaOH Solution A Solution B Solution C Solution D Boiled water I. Which solutions are able to buffer well against acid? Against base? Explain. II. Are any solutions able to buffer well against both acid and base? Explain. III. Do any solutions show little or no buffering ability? Explain. IV. Write equations to show how a buffer made up of equimolar amounts of acetic acid, CH3COOH, and acetate, CH3COO-, would behave when (a) HCl is added and (b) NaOH is added. V. At what point is a buffer solution no longer effective in resisting a pH change when a strong acid is added?