Assignment Ionic Bonding

SCH4C UNIT 1: The Formation of Ionic Compounds Sept. 12, 2014
Part A. The Formation of Ions
1.
Atoms that have lost electrons carry a _______________ charge and are called __________________.
2.
Alternatively, if a neutral atom gains one or more electrons, it becomes _________________ charged and is called an _____________________.
3.
The electrons in the outermost shell of an atom are called _______________________________.
4.
Why will an atom lose or gain one or more electrons?
5.
Sodium, Na, will lose one electron instead of gaining seven electrons to attain a noble gas electron configuration. Why? ______________________________________________________________
6.
Bromine, of the _____________Group has ________________ valence electrons. Bromine will
____________ one electron to become stable. The bromine ion is isoelectronic with ______________.
7.
In general, metals lose electrons and become _____________ while _______________ gain electrons and become anions.
8. Draw the Bohr-Rutherford diagram and Lewis Symbol for Magnesium.
9. Draw the Lewis Symbol for the Magnesium cation. ____________
10. Draw the Bohr-Rutherford Diagram and Lewis Symbol for Chlorine.
11. Draw the Lewis Symbol for the Chlorine anion. ___________
Part B : Ionic Compounds
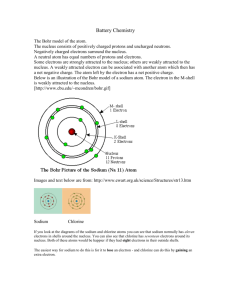
Consider a commonly used seasoning- Table Salt – sodium chloride.
In general, metals lose electrons more easily than other elements and non-metals easily gain electrons.
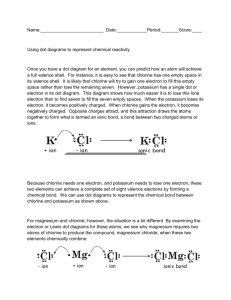
When a sodium atom comes in contact with a chlorine atom, under favourable chemical conditions, the sodium atom will lose its single valence electron to chlorine. Chlorine, with 7 valence electrons, accepts the electron from sodium to attain a stable octet. The transfer allows the sodium atom and chlorine atom to have electron configurations of their nearest Noble Gases, those of lower energies and greater stabilities.
Opposite charges attract. So, the sodium cation (+) and chlorine anion (-) attract strongly in a bond that we call the ionic bond. Ionic bonds form between metals and non-metals.
The crystals of ionic compounds are composed of large numbers of cations and anions bonded together in a recognized crystal lattice. The chemical formula represents the types and ratio of ions in the crystal. The smallest amount having its composition by its chemical formula is called a formula unit.
1. What is the formula unit for: a) sodium chloride; b) magnesium bromide; c) strontium fluoride?
2. Which types of elements form ionic bonds? __________________________________________
3. Predict the charge on the most stable ion formed by the following elements: a) Potassium ___ b) Sulfur ___ c) Iodine ___ d) Barium ___
4. Using Lewis Symbols and the octet rule, illustrate how each of the following pairs of atoms bond: a) Lithium and sulfur b) Calcium and chlorine c) Magnesium and oxygen