Ionic Bonds Practice
advertisement

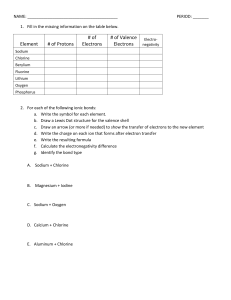
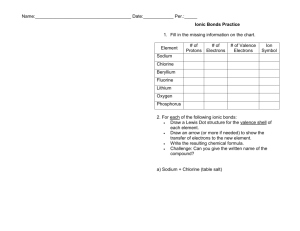
Name:______________________________________ Date:____________ Per.:_____ Ionic Bonds Practice Fill in the missing information on the chart. Element # of Protons Sodium Chlorine Beryllium Fluorine Lithium Oxygen Phosphorus For each of the following ionic bonds: 1. Potassium + Flourine # of Electrons # of Valence Electrons Type of Ion Formed Name:______________________________________ Date:____________ Per.:_____ 2.) Magnesium + Iodine 3.) Sodium + Oxygen 4.) Sodium + Chlorine 5.) Calcium + Chlorine 6.) Aluminum + Chlorine Name:______________________________________ Date:____________ Per.:_____ Covalent Bond Practice 1. Fill in the missing information on the chart. Element # of Protons # of Electrons Carbon Hydrogen Chlorine Helium Phosphorus Oxygen Sulfur Nitrogen 2. For each of the following covalent bonds: 1.) Hydrogen + Hydrogen # of Valence Electrons # of electrons to fill outer shell. Name:______________________________________ Date:____________ Per.:_____ 2.) Hydrogen + Oxygen 3.) Chlorine + Chlorine 4.) Oxygen + Oxygen 5.) Carbon + Oxygen 6.) Carbon + Hydrogen