Review for the Chemistry/Experimental Design Exam
advertisement

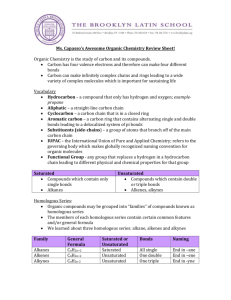
Review for the Chemistry/Experimental Design Exam- H You can to review questions after school from the first two quizzes. You may not take them with you. Here is an outline of what we have covered to date: 1. What are the characteristics of life (see page 4 in the Biology, the Science of Life Section) a. Think about these characteristics: metabolism, homeostasis, reproduction, grow and develop, organized cellular structure, responds to stimuli, adapts to environmental changes (evolution). We have talked about the ones in bold a little. i. Metabolism are chemical reactions. ii. Homeostasis includes maintenance of temperature and pH. iii. Maintenance of temperature is assisted by the high heat capacity of water. iv. Reproduction, growth and development and organized cellular structure all require energy input and chemical reactions, and synthesis of not only chemicals, but living cell. b. Everything we are talking about now, should be able to be linked to the characteristics of life. 2. We then talked about how we “do science” Doing science involves a. Discovery Science b. Hypothesis driven (experimental design) science c. Pure vs. applied science d. Scientific method i. Observations ii. Questions iii. Hypotheses 1. Must be written so that they are TESTABLE and MEASURABLE iv. Experimental Design 1. Includes a measurable dependent variable 2. Levels of an independent variable (which should include one control level) 3. Several variables which are held constant 4. Write good operational definitions to be included in procedures v. You should be able to write results (written) and put data into tables and graphs 1. Results are always observations and data (tables or graphs) vi. You should be able to discuss and draw conclusions ( include inferences) about the data. e. When reading a scientific article you should be able to determine a hypothesis (general) and be able to identify scientific observations and inferences found within the article. 3. We then began the basic chemistry section a. You should be able to differentiate between atoms, molecules and compounds. b. You should be able to differentiate between compounds and mixtures c. You should be able to differentiate between the three types of mixtures d. You should be able to identify atoms that will join to form ionic bonds vs. those that will form covalent bonds e. Differentiate between ionic and covalent bonds. What makes these two bonds different? f. What are chemical formulas? How do you write a chemical reaction? What are three things that a chemical formula can tell us i. Ie 3C6H12O6 1. Elements, number of atoms, number of molecules g. We went over three basic types of reactions (actually four if you count redox). i. What are they? ii. Where would you see them in the human body iii. Why is each important in living things h. How are exothermic and endothermic reactions different. Be able to describe each in terms of initial energy of the reactants, activation energy, and final energy of products. 4. Water a. Is water organic or inorganic? b. What makes a compound organic? i. You should know 5 basic compounds that are organic and found in living things c. What are important inorganic molecules? d. What is the difference between the functions (jobs) of water and the characteristics (properties of water?) e. Based on your webquests, readings and notes, why is the specific heat of water important in terms of climate? f. What is an example of how cohesion g. What types of property are directly related to polarity? h. How do cohesion and adhesion relate to capillary action (and what is capillary action). Does capillary action happen more in small tubes or large tubes? i. Is capillary action more important in blood vessels or plants? j. What types of compounds dissolve in water? i. What won’t dissolve in water? ii. What does Like-dissolves-like mean? iii. When any ionic compound comes apart into two ions and is surround be water molecules, what type of mixture is formed? 5. pH a. How is pH related to water and to living things? How does acid rain affect living things? b. What does pH really measure? c. What are the ranges for acidic and basic d. What is the normal range for “most” of the body. e. Most of the food we eat falls within what pH range? f. Cleaners can be either very acidic or very basic. i. Acids (or acid rain) can actually dissolve ROCK! 1. That’s why they remove “hard water” from glass shower doors g. Your digestive tract has pH that ranges anywhere from about 2 – 8. i. Why do we need the pH to change in so many places? h. How do we keep pH in a “set range” in living things? Provide an example of a human buffer system.