Stoichiometry – Chemical Quantities Notes 1. Stoichiometry – Study
advertisement
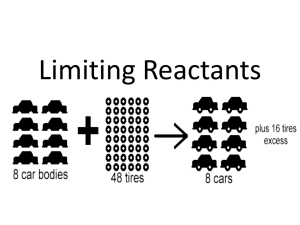
Stoichiometry – Chemical Quantities Notes 1. Stoichiometry – Study of quantitative relationships that can be derived from chemical formulas and chemical equations Mole ─ Mole Relationship o need a balanced equation o Mole Ratio – o The coefficients in a balanced equation give the relative numbers of molecules, as well as, the relative number of moles. CO(g) + 2H2(g) CH3OH(l) o Ex: How many moles of O2 are required to produce 10. moles of CO2? 2 CO + O2 2 CO2 What other relationships do we have for the mole? o o o o We can add these mole relationships on either end of the mole ratio: # unit A x 1 mol A x mol B x __ unit B = # unit B _ unit A _ mol A 1 mol B *A is the GIVEN substance & B is the WANTED substance Mass A – Mole B o Ex: Calculate moles of O2 produced if 2.50 g KClO3 decomposes completely: 2 KClO3 2 KCl + 3 O2 Mass A – Mass B o Ex: Determine the mass of NaCl that decomposes to yield 355 g Cl2 2NaCl 2 Na + Cl2 Mole A – Mass B o Ex: Calculate the number of grams of oxygen required to react exactly with 4.30 mol of propane, C3H8, in the reaction by the following balanced equation: C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) Learning Check - How many grams of water are needed to produce 9.23 moles of oxygen? _____ Na2O2 + _____ H2O _____ NaOH + _____ O2 2. Limiting Reactants & Percent Yield Background Knowledge Check o Label the reactant(s) and product(s) in the following reaction: 2 Mg + O2 2MgO Reactant(s): Product(s): Limiting Reactant Manufacturers of cars and bicycles order parts in the same proportion as they are used in their product. Car manufacturers order four times as many wheels as engines and bicycle manufacturers order twice as many pedals as seats. In the same manner, when chemicals are mixed together so they can undergo a reaction, they are often mixed in stoichiometric quantities – exactly the correct amounts so that all the reactants “run out” at the same time. If the chemicals aren’t mixed to run out at the same time, one of the chemicals will limit or halt the reaction from taking place any further. The reactant that “runs out” or limits the reaction is called the _____________ The reactant that still remains or is extra is called the ___________________ In any stoichiometric problem, where reactants are not mixed in stoichiometric quantities, it is essential to determine which reactant is limiting in order to calculate correctly the amounts of products that will be formed. Analogy: Baking Cookies. A recipe calls for 2 cups of flour for every egg. You have 5 cups of flour and 3 eggs. o What is your limiting ingredient? What is your excess ingredient? Steps for Solving Stoichiometry Problems Involving Limiting Reactants Write and balance the equation for the reaction, if necessary. For each reactant, convert grams reactant to grams product. Compare grams of product: • The smaller grams of product is the _________________ and is the amount of product made • The smaller grams of product came from the ___________________________ • The larger grams of product came from the ____________________________ Limiting Reactant Example : 7.24 moles of Mg and 3.86 moles of O2 react to form MgO. 2 Mg + O2 2MgO How many grams of MgO are formed ? What is the limiting reactant ? What is the excess reactant ? Suppose 2.50 x 104 g of N2 and 5.00 x 103 g of H2 are mixed and reacted to form ammonia. Calculate the mass of ammonia produced when the reaction is run to completion. N2 + 3 H2 2 NH3 How many grams of NH3? What is the limiting reactant? What is the excess reactant? Percent Yield Theoretical yield – amount of product predicted from the amounts of reactants used, calculated from the limiting reactant Actual yield – amount of product actually obtained through experiment Percent yield – comparison of actual and theoretical yield Percent Yield = __________________ X 100 Percent Yield Example: Methanol, CH3OH, can be produced by the reaction between carbon monoxide and hydrogen. Suppose 6.85 x 104 g of CO is reacted with 8.60 x 103 g of hydrogen. CO + 2H2 CH3OH 1. Calculate the theoretical yield of methanol. 2. If 3.57 x 104 g of CH3OH is actually produced, what is the percent yield of methanol? Learning Check - If 1.30 grams of oxygen and 3.10 grams of iron are reacted, then what is the theoretical yield of iron (III) oxide. What is the limiting reactant? If 3.00 grams of iron (III) oxide is produced, then what is the percent yield? Balanced equation: 4 Fe + 3 O2 2 Fe2O3 Theoretical Yield? Limiting Reactant? Percent Yield? Excess Reactant?