Stoichiometry - Fall River Public Schools
advertisement
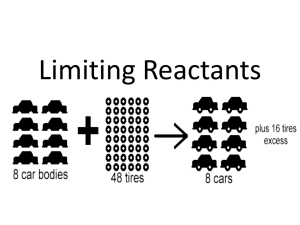
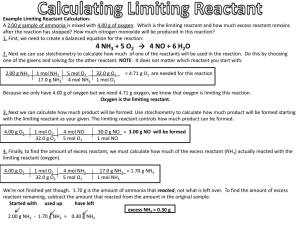
Stoichiometry Chemistry Ms. Piela Stoichiometry • The quantitative study of reactants and products in a chemical reaction ▫ Chemical reactions give info about how molecules combine, and can be used to determine amounts of substances that form Mole to Mole Calculations • When using a chemical equation to determine the amount of the substance, you must relate them by their mole ratios • Example #1: ____ H2 + ____ O2 → ____ H2O If you start with 4 moles of O2 how many moles of H2O would form? Mole to Mole Calculations • Example #2: ___ Fe + ____ S → ___Fe2S3 • How many moles of iron (III) sulfide, Fe2S3, forms if the reaction initially starts with 8 moles of iron, Fe? Mass to Mass Calculations • Example #1: ___ H2 + ___ O2 → ___ H2O If 2.4 g of O2 are reacted with excess H2 gas, how many moles of H2O would form? 0.15 mol H2O Mass to Mass Calculations • Example #2: ___ H2 + ___ O2 → ___ H2O In the above equation, if I started with 3 grams of H2, how many grams of H2O would form? 26.8 g H2O Mass to Mass Calculations • Example #3: _ NH3 (g) + _ CO2 (g) → _ (NH2)2CO(aq) + _H2O(l) When 2.00 grams of ammonia, NH3, react with excess CO2, what mass of water (in grams) is formed? 1.06 g H2O Limiting Reactant • The reactant used up first in a chemical reaction ▫ Also called limiting reagent • After a reaction, excess reactants are left ▫ These are reactants present in greater quantities that remain after a reaction takes place Limiting Reactant • Chair analogy: It’s your lucky day! Mr. Nelson has hired you to make chairs! The chairs follow the “reaction”: 4 legs + 1 seat + 10 screws → 1 chair Here are the supplies you have: 10 legs, 5 seats, and 100 screws. How many chairs be made? What is the limiting reactant? Limiting Reactant Simulation • http://phet.colorado.edu/en/simulation/reacta nts-products-and-leftovers Limiting Reactant •The limiting reactant can be found by determining which of the starting amounts yields the least amount of product Limiting Reactant Example Problem ▫ Sodium chloride can be prepared by the reaction of sodium with chlorine gas: 2Na (s) + Cl2 (g) 2NaCl (s) ▫ Suppose that 6.70 mol Na reacts with 3.2 mol Cl2 How many moles of NaCl will be formed in this reaction? 6.4 mol NaCl What is the limiting reactant? Cl2 Limiting Reactant Practice Problem • 2.70 mol C2H4 is reacted with 6.30 mol of O2. Calculate the amount in moles of water that forms, and identify the limiting reactant. C2H4 (g) + 3O2 (g) 2CO2 (g) + 2H2O (g) 4.2 mol H2O Limiting Reactant Example Problem • Urea is prepared by reacting ammonia with carbon dioxide: 2 NH3 (g) + CO2 (g) → (NH2)2CO(aq) + H2O(l) In one process, 637.2 g of NH3 are treated with 1142 g of CO2. Calculate the mass of urea, (NH2)2CO, formed. 1122 g urea Limiting Reactant Practice Problem • Calculate the number of grams of NH3 produced by the reaction of 5.40 g of hydrogen with 8.25 g of nitrogen. The balanced equation is: N2 + 3 H2 2 NH3 9.97 g NH3 Percent Yield • Theoretical yield is the amount of product that would result if all the limiting reagent reacted • Actual yield is the amount actually obtained in lab from a reaction ▫ Actual yield is almost always less than theoretical Percent Yield • Percent yield describes proportion of actual yield to theoretical yield. Percent Yield actual yield theoretica l yield x 100 % Percent Yield Example Problem • When 84.8 g of iron (III) oxide reacts with an excess of carbon monoxide, 54.3 g of iron is produced. What is the percent yield of this reaction? Fe2O3 (s) + 3CO (g) 2 Fe (s) + 3CO2 (g) 91.6% Percent Yield Practice Problem • If 25 grams of carbon dioxide gas can be produced in the following reaction, how many grams of sodium hydroxide should be produced? If 10.7 grams of sodium hydroxide are actually produced, what is the percent yield? NaHCO3 NaOH + CO2 22.7 g NaOH / 41.1%