Limiting Reactants
advertisement
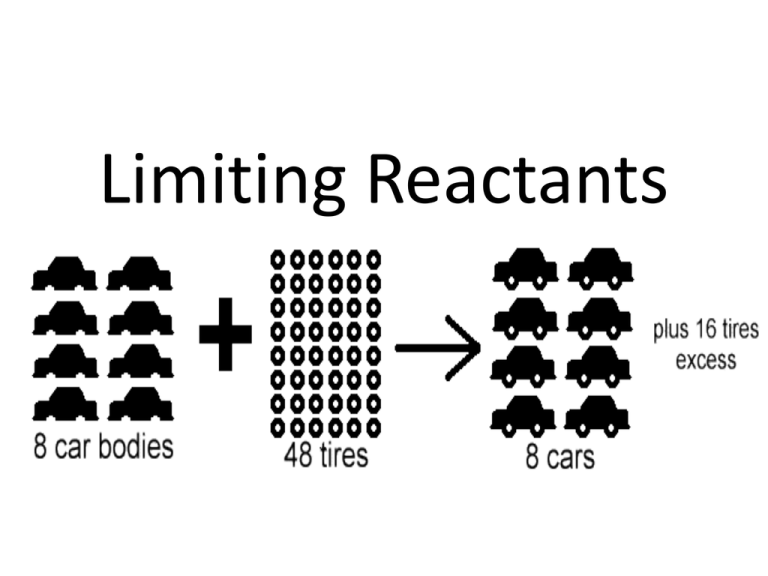
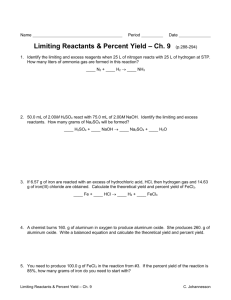
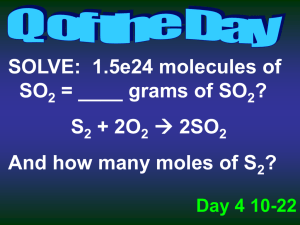
Limiting Reactants Reactants Products • Limiting Reactant: Determines the amount of products that can be made • Excess Reactant : Left over reactants Identifying the Limiting Reactant • Convert each reactant to grams of a product using stoichiometry • The reactant that makes the least amount of products is the limiting reactant Sample Problem Which is the limiting reactant if 25.0 grams of phosphorus reacts with 50.0 grams of oxygen? P4 + 5O2 P4O10 P4 is limiting Sample Problem A 2.00 g sample of ammonia is mixed with 4.00 g of oxygen. How much NO is produced and which is the limiting reactant? 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) Sample Problem An alkaline battery produces energy according to the following reaction. Determine the limiting reactant if 25.0 g of Zn and 30.0 g of MnO2 are used. What mass of Zn(OH)2 is produced? Zn + 2MnO2 + H2O Zn(OH)2 + Mn2O3 MnO2 is limiting Excess Reactants • The amount of excess reactants can be determined by using stoichiometry to find the amount of reactants that is needed to react with the limiting reactant. Yield • The theoretical yield represents the maximum amount of product that can be formed from given amounts of reactants • The actual yield is the amount of product that actually forms when the reaction is carried out (in the laboratory). It is considered as an experimental value. Percent Yield A ratio of actual yield to theoretical yield Why might percent yield be important? Percent yield is important, because… • Higher yield=less waste • Less waste= lower cost for products Percent Error: how much error there is between actual and theoretical yield Sample Problem Aluminum hydroxide is often present in antacids to neutralize stomach acid (HCl). If 14.0 g of aluminum hydroxide is present in an antacid tablet, determine the theoretical yield of aluminum chloride produced when the tablet reacts with stomach acid. If the actual yield of aluminum chloride is 22 g, what is the percent yield? Al(OH)3 + 3HCl AlCl3 + 3H2O Al(OH)3 + 3HCl AlCl3 + 3H2O What is the percent error?