L - Zn_HCl lab.13
advertisement

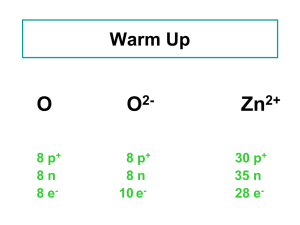
The Law of Definite Proportions: an Application Chem. 319 Ms. Hobbie Some changes have been made. LET THEM DO THE CALCS TOGETHER IN CLASS! Then let them write an introduction: “goals and theory” in their own words, and you can grade them on this and their “guidance” through the calculations. Background The first quantitative work in chemistry was done by Antoine and Marie-Ann Lavoisier in the mid 18th century. This scientific couple confirmed the law of conservation of mass by performing careful experiments that showed that, although matter can change its state in a chemical reaction, the total mass of matter is the same at the end as at the beginning of every chemical change. The couple also added to our understanding of compounds by stating the law of definite proportions. This law states that when a compound is formed from the chemical reaction of two elements, the elements combine in definite, fixed proportions by mass. Objectives In this lab work, you’ll react zinc and zinc oxide each with hydrochloric acid. The reaction of either zinc or zinc oxide with hydrochloric acid produces zinc chloride. You will use your results to experimentally determine how much greater or smaller a zinc atom is to a chlorine atom, and a zinc atom is to an oxygen atom. Using the periodic table, you will then be able to identify the correct chemical formulas for the compounds made from these elements. Practicing the calculations In Lavoisiers’ time, the mass of a single hydrogen atom was simply defined as 1.00 because it could not be directly measured. The mass of other elements could be determined relative to hydrogen using chemical reactions with a known reactant mass and a guess at the formula of the compound created—this entire scheme hinged on correctly guessing the formula of the compound. To see how this works, perform the following: Consider the synthesis of water, which is formed in the explosive reaction of hydrogen and oxygen. Let’s imagine that in a certain experiment 3.06 grams of hydrogen was reacted with excess oxygen (i.e. with enough oxygen to ensure that all the hydrogen reacts) and that 27.24 grams of water was formed. What mass of oxygen reacted with the hydrogen? The Lavoisiers assumed the formula for water was HO. Using your data and this formula, calculate how many times greater in mass an oxygen atom is than a hydrogen atom. How does your answer change when you use the correct formula for water, H 2O? Now look at the periodic table. It contains information about what we now know to be the “true” masses of the elements.1 Let’s assume the mass of a hydrogen atom is one “unit.” What value in the oxygen “box” seems to represent mass of oxygen for the correct formula for water, thus must be the correct mass of oxygen? Now take a look at the same location within the hydrogen “box”… was it okay to assume the mass of a hydrogen atom is one unit? Procedural Thoughts and Hints This is a quantitative investigation. Consider carefully all of the masses you need to record. A Sharpie is useful for labeling glassware. You will need to measure the mass of two empty beakers, one for each experiment. You should use somewhere between 0.45 and 0.55 grams each of zinc and zinc oxide. You will be provided with an aqueous solution of 6.0 M hydrochloric acid, HCl. About 15 mL should be excess, that is, enough to complete each reaction. 6.0 M is rather concentrated, so handle this carefully 1 The units of mass on the periodic table for individual atoms are atomic mass units. HCl is a gas that dissolves readily in water. After the reaction, you can drive off the excess HCl and evaporate away the water by placing the reaction flask on a hot plate. Be thoughtful about limiting error as much as possible. Data and Observations Use a separate piece of paper to record all of your data measurements as well as any observations you have as the reactions proceed. Provide data table? Or make a data table on the board on the day they do their calcs in class. Four rows, mass of beaker, mass of reactant (Zn or ZnO), mass of residue + beaker (zinc chloride), mass of zinc chloride (a calculated value) Analysis and Results Using only lab data, determine two possble mass ratios of a zinc atom to a chlorine atom (i.e. how many times greater or smaller the mass of a zinc atom is than a chlorine atom) by first assuming that the formula of the compound is ZnCl and then assuming the formula of the compound is ZnCl2. Do the same for zinc and oxygen, again by first assuming that the formula of the compound is ZnO and then assuming the formula of the compound is ZnO2. Hint: you will find the ratio of zinc to zinc chloride from reaction #1 helpful here… Use the masses listed on the periodic table to identify the correct formulas for these two compounds. Write a balanced chemical equation for each of these reactions, including states. You will have to figure out where the extra atoms go… Should this be an extension question, no help from others? Use your work on this analysis to experimentally determine the correct mass ratio of Cl to O. The above-determined formulas, the balanced equations, and/or some algebra might be useful here. Explain your answer. Error Analysis Scientific errors are errors that are inherent to the method involved with the investigation and occur even when the investigators have “done their best” to limit any factors that would alter the result. True scientific errors are either random or systematic, that is, they either have an unpredictable effect on the numerical result or can be predicted to have a specific influence on the numerical result, either making it too large (positive deviation) or too small (negative deviation). NOTE: Human errors, commonly called “blunders” are the result of poor technique by the experimenters. These are NOT considered scientific error and should not be referred to in the Error Analysis. In fact, if you know a “blunder” has occurred you should start over! One specific error inherent to this investigation is the spattering of solid zinc chloride out of the beakers. (Do NOT consider the possibility of spattering into beakers from “neighbors” on the hot plate.) What effect would this spattering error have on the results of your reaction of Zn with HCl? Would this error be considered random or systematic? If systematic, would it result in a positive or a negative deviation in your calculated ratio of zinc mass and chlorine mass? Fully defend your answer, providing a specific explanation that “walks your reader through” the effect of this error on the calculations that lead to your numerical result. What effect would this spattering error have on the results of your reaction of ZnO with HCl? Would this error be considered random or systematic? Provide the same full defense of your answer. Thought Questions NOTE: read section 2.1!!! 1. What evidence do you have from your work in the lab that the Zn/HCl mixture underwent a chemical change, and not just a physical change? Be sure to explain how you distinguish between these two in your answer. 2. What evidence do you have from your work in the lab that the zinc oxide/HCl mixture underwent a chemical change, and not just a physical change? ________________________________________________________________________ Hand-In Assignment “Goals and theory” or back to presenting an abstract? Data table, well-labeled with units on every recorded measurement – hand written. Analysis and Results – hand-written, legible. Provide a heading or a brief explanation of each step of any calculations you present, and be sure to include units and a label on every numerical value. Attach TYPED answers to the Error Analysis and Thought Questions. Collaboration: You may compare your analysis and results with your partner and with others in the class, but the written analysis itself should be your own work in your own words.