Powerpoint: Chemical Reactions
advertisement
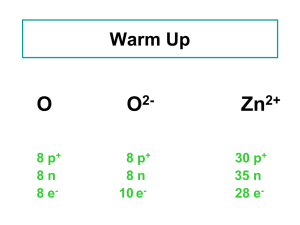
Chemical Reactions Standards Conservation of Matter and Stoichiometry 3a. Students know how to describe chemical reactions by writing balanced equations Objectives Identifying the reactants, products and other parts of chemical equations Write word equations from a chemical reaction description Write chemical equations from word equations Identify and include solid (s), liquid (l), gas (s), and/or aqueous (aq) in chemical equations Identify and write the seven diatomic elements Definitions Chemical equation: a precise detailed accounting of both the identities and amounts of reactants consumed and products formed in a reaction Identities: all participants in reaction get named Amounts: how much is specified in equation, (moles) Reactants: the starting substance(s) in a chemical reaction Product: substance(s) formed in a chemical reaction Definitions Word equation: description of a reaction without using chemical formulas Reactants: what you start with Products: what is made Definitions Catalyst: a substance that speeds up a reaction without being used up; enzymes good example Aqueous: dissolved in water (aq) Diatomic: two of the same atom naturally bound together Writing a Word Equation Hydrogen reacts with oxygen to form water Word Equation: hydrogen + oxygen → water Reactants Chemical Equation: H2 + O2 →H2O Product Diatomic Elements Diatomic: two of the same atom naturally bound together Hydrogen and Oxygen and some others exist bound together. They are always found as a pair, so when you write them write them as H2, O2. Diatomic Elements “7” Write the Word & Chemical Equation… Calcium metal reacts with chlorine gas to produce calcium chloride Calcium + chlorine → calcium chloride Ca + Cl2 → CaCl2 Write the Word & Chemical Equation… When zinc metal reacts with oxygen gas, solid zinc oxide is formed. Zinc + oxygen → zinc oxide Zn + O2 → ZnO Write the Word and Chemical Equation… Carbon dioxide gas and water are produced when methane gas is burned in oxygen. Methane + oxygen → carbon dioxide + water CH4 + O2 → CO2 + H20 Write the Word & Chemical Equation… When zinc metal is placed in a solution of hydrochloric acid, hydrogen gas and a zinc chloride solution are formed. Zinc + hydrochloric acid → hydrogen gas + zinc chloride Zn(s) + HCl (aq) → H2 (g) + ZnCl2 (aq) Rules for balancing 1. Must have same # of each atom on both sides of the equation 2. Must use coefficients to balance, can not add subscripts Balance the Equation Calcium metal reacts with chlorine gas to produce calcium chloride Calcium + chlorine → calcium chloride Ca + Cl2 → CaCl2 Balance the Equation When zinc metal reacts with oxygen gas, solid zinc oxide is formed. Zinc + oxygen → zinc oxide Zn + O2 → ZnO Balance the Equation Carbon dioxide gas and water are produced when methane gas is burned in oxygen. Methane + oxygen → carbon dioxide + water CH4 + O2 → CO2 + H20 Balance the Equation When zinc metal is placed in a solution of hydrochloric acid, hydrogen gas and a zinc chloride solution are formed. Zinc + hydrochloric acid → hydrogen gas + zinc chloride Zn(s) + HCl (aq) → H2 (g) + ZnCl2 (aq) WORKSHEET a END