Exercise8_solutions
advertisement
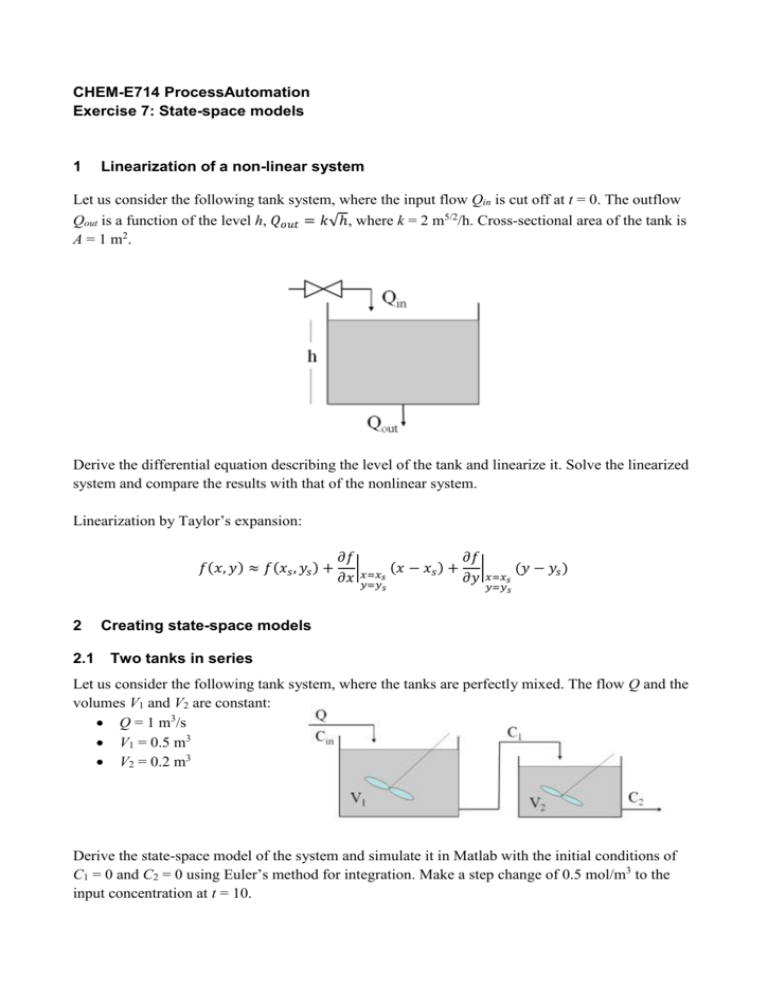
CHEM-E714 ProcessAutomation Exercise 7: State-space models 1 Linearization of a non-linear system Let us consider the following tank system, where the input flow Qin is cut off at t = 0. The outflow Qout is a function of the level h, 𝑄𝑜𝑢𝑡 = 𝑘√ℎ, where k = 2 m5/2/h. Cross-sectional area of the tank is A = 1 m2 . Derive the differential equation describing the level of the tank and linearize it. Solve the linearized system and compare the results with that of the nonlinear system. Linearization by Taylor’s expansion: 𝑓(𝑥, 𝑦) ≈ 𝑓(𝑥𝑠 , 𝑦𝑠 ) + 𝜕𝑓 𝜕𝑓 |𝑥=𝑥 (𝑥 − 𝑥𝑠 ) + | (𝑦 − 𝑦𝑠 ) 𝜕𝑥 𝑦=𝑦𝑠 𝜕𝑦 𝑥=𝑥𝑠 𝑠 2 2.1 𝑦=𝑦𝑠 Creating state-space models Two tanks in series Let us consider the following tank system, where the tanks are perfectly mixed. The flow Q and the volumes V1 and V2 are constant: Q = 1 m3/s V1 = 0.5 m3 V2 = 0.2 m3 Derive the state-space model of the system and simulate it in Matlab with the initial conditions of C1 = 0 and C2 = 0 using Euler’s method for integration. Make a step change of 0.5 mol/m3 to the input concentration at t = 10. 2.2 Isothermal reactor Consider the following reaction scheme, taking place in an isothermal reactor: k1 k2 A B C k3 2 A D with the following molar rates of formation: k1 = 5/6 min-1 k2 = 5/3 min-1 k3 = 1/6 l/mol min Assuming that the feed stream contains only component A, with initial concentration CA0 = 10, define the state-space model and study the formation of B in the following cases: The dilution rate is Fs/V = 4/7 min-1; The dilution rate is Fs/V = 2,8744 min-1; This reaction scheme was presented by Van de Vusse (1964) and it turned out that this type of system can have significantly different input/output characteristics, depending on the chosen operating condition. Solutions 1 Linearization Before linearizing the previous function, we need to find the steady-state operating point. That is dh obtained by solving the dynamic equation for = 0, in order to get hss. Using a truncated Taylor dt series expansion, we find: Q 1 k k h hss f (h, Qin ) in,ss hss Qin Qin, ss A 2 A hss A A The first term on the right hand side is zero, because the linearization is about a steady-state point. That is: Qin,ss k dh hss 0 hss ,Qin , ss dt A A We can now write: d (h hss ) 1 k h hss Qin Qin, ss dt A 2 A hss and using deviation notation (h’ = h - hss and Q’ = Q - Qss), and dropping the ≈ dh ' 1 ' k Q in h' dt A 2 A hss Taking the Laplace transform, we get: 1 k s H ( s) Qin ( s) H ( s) A 2 A hss and then, the transfer function: 1 H ( s) A k Qin ( s ) s 2 A hss Using the numerical values, we get: G (s) 1 s 1 There is a step-like change in Q in' = -2m3/h, that in Laplace domain is Qin(s) = -21/s . Follows: H ( s) G ( s) Qin ( s) H ( s) 1 2 s 1 s Taking the inverse transform, we get the dynamic behavior: L -1 H (s) L -1 2 s ( s 1) h ' (t ) From the table, we get: h’(t) = -2(1 – e-t) Finally, h(t) = hss + h’(t) = 2et - 1 (1) Differential equation Solving the differential equation: dh 1 (Qin k h ) dt A with an initial value h(0) = 1m and Qin = 0 m3/h, h = 2 m5/2/h, A = 1m, we get: dh 2 h dt and integrating: h(t ) (1 t ) 2 (2) To compare the different results, we plot the curves in Equation (1) and (2), in Figure 2.2. 2.1 Two tanks in series In order to define the system of differential equations for the given system, we consider the mass balance equations around the two reactors and we get: dC V1 1 Q(C in C1 ) dt dC 2 V2 Q(C1 C 2 ) dt Substituting the numerical values, we have: dC1 2C in 2C1 dt dC 2 5C1 5C 2 dt That can be written as a state space model: 0 C1 2 C1 2 C 5 5 C 2 0 in C 2 which has the general form: x = Ax + Bu where 2 0 A= 5 5 2 B= 0 The state and the input vectors are (notice that the input is a scalar): x x = 1 and u = C in x2 It should be noticed that for linear systems, we can write the deviation variable model directly from the physical model, skipping the intermediate steps. The additional equation that is normally associated with a state model is y = Cx + Du where y is the vector of output variables Generally, output variables are variables that can be measured (at least conceptually) or are of particular interest in a simulation study. Here, we consider the output from the second reactor as the only output for our system and we get: y = 0 1 x To define the transfer function for the system, we need to take first the Laplace transform of the system: sC1(s) = -2C1(s) + 2Cin(s) sC2(s) = 5C1(s) - 5C2(s) That can be also written as: sX(s) = AX(s) + BU(s) Y (s) = CX(s) Now, we can act in two ways: Calculating the transfer function directly from the system equations: G ( s) G( s) 2 Gin (s) we get: G( s) 10 ( s 2)( s 5) Calculating the transfer function using matrices: Y (s) G (s) C (sI - A)-1 B U (s) s 2 G( s) 0 1 5 0 s 5 1 2 0 we get: G( s) 10 ( s 2)( s 5) The weighting function is the inverse transform of the function G(s) (from the table): 1 1 G( s) g (t ) (e bt e at ) ( s a)( s b) a b that is: 10 10 G( s) g (t ) (e 2t e 5t ) ( s 2)( s 5) 3 The step input is the most common input forcing function; it is used to solve dynamic problems where a sudden change in the input variable occurs. In the Laplace domain, if A is the step amplitude, we have: A L s Considering a unit step, we get: 1 Y (s) G(s) s 10 1 Y (s) ( s 2)( s 5) s The step response is the inverse of the function Y (s): t 1 0 f (t ) s Ff (t ) F (s) 5 2 y (t ) 1 e 2t e 5t 3 3 Matlab The transfer function using Matlab can be easily found, with the routine ss2tf that can be used to convert a state-space model to a transfer function model. After entering the matrices A, B, C and D, the command: [num,den] = ss2tf(A,B,C,D,iu) will generate the numerator and denominator Laplace domain polynomials from the transfer function between input number iu and the outputs. The Matlab step function assumes a deviation variable form (the initial conditions are zero). The command is: step(SYS) which plots the step response of the LTI model SYS 2.2 Isothermal reactor Assuming constant density and constant volume, we first note that the overall mass balance simply gives F = F0. Component A Balance dC A F (C A0 C A ) k1C A k 3C A2 dt V (3) Component B Balance dC B F (4) C B k1C A k 2 C B dt V Component C Balance dCC F (5) CC k 2 C B dt V Component D Balance dC D F 1 (6) C D k 3C A2 dt V 2 Since we are interested in the production of the component B and since Equations (3) and (4) do not depend on CC and CD, we need to solve only those Equations. Steady-State Solving for the steady-state for Equation (3), we find a quadratic equation in CA and solving it (using only the positive root): C As F F F 1 ( ( k1 s ) ( k1 s ) 2 4 k 3 s C A 0 ) 2k 3 V V V and solving for CB: C Bs k1C As Fs k2 V State-Space Model We linearize the nonlinear modeling equations to find the state space form, where the state, input and output vector are, deviation form: C C As C C As F F x= A , u = s , y= A V V C B C Bs C B C Bs The elements of the state-space A are found as Aij f i x j , where f1 is the balance of x1 = CA and f2 is the balance of x2 = CB: a11 F f1 s k1 2k 3C As x1 V b11 f1 C A0 s C As u1 a12 f1 0 x 2 b21 f 2 C Bs u1 a 21 f 2 k1 x1 a 22 F f 2 s k2 x 2 V The state-space model is: Fs k1 2k 3C As A = V k1 , B = C A0 s C As C Fs Bs k2 V 0 Case 1 Considering a dilution rate of Fs/V = 4/7 min-1, we obtain the following steady-state concentration: CAs = 3 mol/liter CBs = 1,117 mol/liter which yield to: A = 2,4048 0 ,B= 0,8333 2,2381 7,0000 1,1170 The process transfer function relating input 1 and output 2 is: G ( s) 1,117 s 3,1472 s 4,6429s 5,3821 2 Case 2 Considering a dilution rate of Fs/V = 2,8744 min-1, we obtain the following steady-state concentration: CAs = 6,0870 mol/liter CBs = 1,117 mol/liter which yield to: A = 5,7367 0 , B = 3,9130 1,1170 0,8333 4,5411 The process transfer function relating input 1 and output 2 is: G ( s) 1,117 s 3,1472 s 10,2778 26,0508 2