OHAs and other illness advice
advertisement

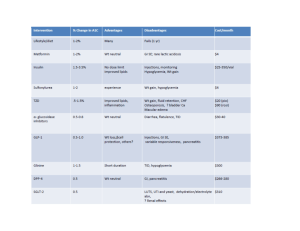
BIGUANIDES (Metformin immediate/slow release) Metformin is the most commonly used oral hypoglycaemic agent. It works by i) reducing hepatic glucose production, ii) increasing peripheral utilisation of glucose in muscle and fat tissues (increases sensitivity of insulin), iii) decreasing intestinal absorption of glucose and iv) decreasing insulin requirements for glucose disposal. The most common side-effects include GI side effects, malabsorption of vitamin B12 and occasionally rash. A rare, but potentially very serious side-effect is lactic acidosis therefore metformin should be stopped if e-GFR is less than 30mL/minute/1.73m2. Additionally, withdraw or interrupt treatment in those at risk of tissue hypoxia or sudden deterioration in the renal function, such as those with dehydration, severe infection, shock, sepsis, acute heart failure. SULFONYLUREAS (gliclazide, glibenclamide, glimepride, glipizide, tolbutamide) Sulfonylureas work by increasing pancreatic insulin secretion. They may also improve insulin sensitivity in peripheral tissues and decrease hepatic glucose output. Hypoglycaemia is the most frequent and serious adverse effect. All patients who take sulfonylureas are at risk but especially the elderly, those with renal or hepatic impairment and those receiving interacting drugs. THIAZOLIDINEDIOINES/GLITAZONES (Pioglitazone) They work by increasing the sensitivity of peripheral tissues to insulin and reducing the hepatic glucose output. They are used in patients with T2DM in addition to metformin, sulfonylureas or combination of these two groups. They cause hypoglycaemia less often than sulphonylureas. However, incidence of heart failure is increased, especially when they are used in combination with insulin in patients with predisposing factors, e.g. previous myocardial infarction. Patients who take pioglitazone should be closely monitored for signs of heart failure; treatment should be discontinued and the diabetes team should be informed if any deterioration in the cardiac status occurs (e.g. pulmonary oedema, peripheral oedema, weight gain). This class of medications causes bone loss and further increases facture risk, placing thiazolidinediones in the category of drugs causing secondary osteoporosis. Please inform the diabetes team if you meet a patient with new or established osteoporosis who is on thiazolidinediones. DIPEPTIDYL PEPTIDASE-4 (DPP-4) INHIBITORS (Sitagliptin, linagliptin, saxagliptin, vildagliptin) They inhibit DPP-4 to increase insulin secretion and lower glucagon secretion. They are used in combination with other oral hypoglycaemic agents or insulin or, less commonly, as monotherapy for patients with T2DM. The most common side-effects include gastrointestinal issues, including nausea, diarrhoea and stomach pain and flu-like symptoms. Treatment should be withheld in these cases and the diabetes team should be informed. DPP-4 inhibitors have been linked with an increased risk of pancreatitis therefore treatment should be withheld in patients presenting with severe nausea, vomiting and/or abdominal/epigastric pain until amylase is urgently checked. GLUCAGON-LIKE PEPTIDE-1 (GLP-1) AGONISTS (Exenatide, liraglutide, lixisenatide) They bind to, and activate, the GLP-1 receptor to i) increase insulin secretion, ii) supress glucagon secretion, iii) slow gastric emptying and iv) possibly promote satiety by hypothalamic receptors. They are used in obese patients with T2DM as subcutaneous injections in combination with oral hypoglycaemic agents. Their common side effects include gastro-intestinal disturbances (nausea, vomiting, diarrhoea, dyspepsia and abdominal pain) and they can also enhance sulfonylureainduced hypoglycaemia. Less commonly, GLP-1 agonists have been linked with an increased risk of pancreatitis therefore treatment should be withheld in patients presenting with severe nausea, vomiting and/or abdominal/epigastric pain until amylase is urgently checked. In these cases, please notify the inpatient diabetes team. SODIUM-GLUCOSE CO-TRANSPORTER 2 (SGLT2) INHIBITORS (Dapagliflozin, Canagliflozin, Empagliflozin) They reversibly inhibit SGLT2 in the renal proximal convoluted tubule to reduce glucose reabsorption and increase urinary glucose excretion. They are used in combination with other oral hypoglycaemic agents or insulin in the treatment of T2DM or, very rarely, as monotherapy. Their obvious side effects include genital or urinary tract infections or thrush. They can cause hypoglycaemia when used in combination with sulfonylurea or insulin. Recently there have been reports of ketoacidosis in patients on these drugs. The mechanism suggested is not confirmed and more research is needed, however it is recommended that patients on SGLT2 inhibitors whose clinical presentation on admission raises the possibility of acidosis should have venous bicarbonate and serum or urine ketones tested. Several of these cases reported were of euglycaemic diabetic ketoacidosis (ketoacidosis with normal glucose) therefore a normal glucose level does not exclude DKA. If the diagnosis of DKA is confirmed, the medication should be stopped and the patient should be treated as per guidelines for DKA. In these cases, please refer the patient to the inpatient diabetes team.